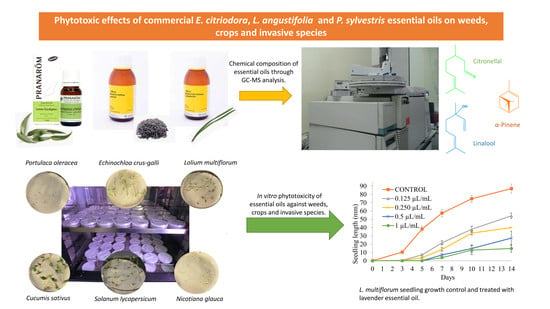
Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of E. citriodora, L. angustifolia, and P. sylvestris Essential Oils
2.2. Seed Germination Inhibition of P. oleracea, L. multiflorum, E. crus-galli, Tomato, Cucumber and N. galuca with E. citriodora, L. angustifolia and P. sylvestris Essential Oils
2.3. Seedling Growth Inhibition of P. oleracea, L. multiflorum, E. crus-galli, Tomato, Cucumber and N. glauca with E. citriodora, L. angustifolia, and P. sylvestris Essential Oils
3. Materials and Methods
3.1. Essential Oils
3.2. Weeds, Food Crops, and Invasive Species Seeds
3.3. Gas Chromatography–Mass Spectrometry Analysis
3.4. In Vitro Assays: P. oleracea, L. multiflorum, E. crus-galli, Tomato, Cucumber, and N. glauca Seed Germination and Seedling Growth with Essential Oils
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, L.; Moghaddam, M. Essential oils: Biological activity and therapeutic potential. In Therapeutic, Probiotic and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: London, UK, 2018; pp. 167–176. [Google Scholar]
- Morsy, N.F.S. Chemical structure, quality indices and bioactivity of essential oil constituents. In Active Ingredients from Aromatic and Medicinal Plants; InTech: London, UK, 2017; pp. 175–206. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L. Essential oils chemistry. In Bioactive Essential Oils and Cancer; Sousa, D.P., Ed.; Springer: Basel, Switzerland, 2015; pp. 19–28. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Jamoussi, B. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weeds management. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 96–114. [Google Scholar]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017, 28, 1168–1180. [Google Scholar] [CrossRef] [Green Version]
- Fouad, R.; Bousta, D.; El Ouali, A.; Chahdi, F.O.; Amri, I.; Jamoussi, B.; Greche, H. Chemical composition and herbicidal effects of essential oils of Cymbopogon citratus (DC) Stapf, Eucalyptus cladocalyx, Origanum vulgare L. and Artemisia absinthium L. cultivated in Morocco. J. Essent. Oil Bearing Plants 2015, 18, 112–123. [Google Scholar] [CrossRef]
- Matković, A.; Marković, T.; Vrbničanin, S.; Sarić-Krsmanović, M.; Božić, D. Chemical composition and in vitro herbicidal activity of five essential oils on Johnson grass (Sorghum halepense [L.] Pers.). Lek. Sirovine 2019, 38, 44–50. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef]
- Batish, D.; Setia, N.; Singh, H.P.; Kohli, R. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Prot. 2004, 23, 1209–1214. [Google Scholar] [CrossRef]
- CABI, Invasice Species Compendium. Portulaca oleracea (purslane). 2018. Available online: https://www.cabi.org/isc/datasheet/43609 (accessed on 24 March 2019).
- CABI, Invasice Species Compendium. Lolium multiflorum (Italian ryegrass). 2018. Available online: https://www.cabi.org/ISC/datasheet/31165 (accessed on 24 March 2019).
- Perez-Jones, A.; Park, K.W.; Colquhoun, J.; Mallory-Smith, C.; Shaner, D. Identification of glyphosate-resistant Italian ryegrass (Lolium multiflorum) in Oregon. Weed Sci. 2005, 53, 775–779. [Google Scholar] [CrossRef]
- Tehranchian, P.; Nandula, V.K.; Matzrafi, M.; Jasieniuk, M. Multiple herbicide resistance in California Italian ryegrass (Lolium perenne ssp. multiflorum): Characterization of ALS-inhibiting herbicide resistance. Weed Sci. 2019, 67, 273–280. [Google Scholar] [CrossRef]
- CABI, Invasice Species Compendium. Echinochloa crus-galli (barnyard grass). 2018. Available online: https://www.cabi.org/isc/datasheet/20367 (accessed on 24 March 2019).
- Thomas, J.; El-Sheikh, M.; Alfarhan, A.; Alatar, A.; Sivadasan, M.; Basahi, M.; Al-Obaid, S.; Rajakrishnan, R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid Environ. 2016, 127, 53–65. [Google Scholar] [CrossRef]
- Ayenew, A.; Faris, G.; Seifu, A.; Merawi, E.; Seboka, N.; Misganaw, M.; Bekeke, T. Impact and status of invasive alien plant species (IAPS), Nicotiana glauca, in Eastern and Southern Zones of Tigray regional state, Ethiopia. Biodivers. Int. J. 2018, 2, 351–355. [Google Scholar]
- Upasani, R.R.; Barla, S. Herbicide resistance in weeds it’s management. J. Pharmacogn. Phytochem. 2018, 810–815. [Google Scholar]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Qasem, J.R. Herbicides applications: Problems and considerations. In Herbicides and Environment; Kortekamp, A., Ed.; InTech: London, UK, 2011; pp. 643–664. [Google Scholar]
- Lamb, R.D.; Johnson, M.D. Agricultural compositions and applications utilizing essential oils. United States Patent US 9.949.490 B2, 2018. [Google Scholar]
- Fernandez, L.; Campbell, B.; Huang, H.; Koivunen, M.; Marrone, P.G. Natural herbicide containing lemongrass essential oil. United States Patent Application US 20090099022 A1, 2019. [Google Scholar]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Aragão, F.B.; Palmieri, M.J.; Ferreira, A.; Costa, A.V.; Queiroz, V.T.; Pinheiro, P.F.; Andrade-Vieira, L.F. Phytotoxic and cytotoxic effects of eucalyptus essential oil on lettuce (Lactuca sativa L.). Allelopath. J. 2015, 35, 259–272. [Google Scholar]
- Ibáñez, M.D.; Blázquez, M.A. Tea tree and wintergreen essential oils in the management of the invasive species Cortaderia selloana and Nicotiana glauca. J. Plant Prot. Res. 2019, 59, 1–10. [Google Scholar] [CrossRef]
- Tolba, H.; Moghrani, H.; Benelmouffok, A.; Kellou, D.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition, antifungal activity. J. Mycol. Med. 2015, 25, e128–e133. [Google Scholar] [CrossRef]
- Tolba, H.; Moghrani, H.; Aboun, A.; Maachi, R. Essential oil of Algerian Eucalyptus citriodora: Chemical composition and antimicrobial activities. Nat. Technol. J. 2018, 18, 19–27. [Google Scholar] [CrossRef]
- Davies, J.H.; Moses, J. Insect repellent composition and method of use. US Patent US2019/0037840 A1, 2019. [Google Scholar]
- Abril-Sánchez, C.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 2019, 219, 72–78. [Google Scholar] [CrossRef]
- De Rapper, S.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evidence Based Complement. Altern. Med. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Simsek, M.; Duman, R. Investigation of effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacognosy Res. 2017, 9, 234–237. [Google Scholar] [CrossRef] [Green Version]
- Özek, T.; Tabanca, N.; Demirci, F.; Wedge, D.E.; Can Baser, K.H. Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod. 2010, 4, 180–192. [Google Scholar]
- Yuan, C.; Wang, Y.; Liu, Y.; Cui, B. Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2019, 130, 104–110. [Google Scholar] [CrossRef]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.; Mihajilov-Krstev, T.; Stojanović-Radić, Z.Z.; Cvetković, V.J.; Mitrović, T.L.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2018, 111, 55–62. [Google Scholar] [CrossRef]
- Da Silva, A.C.R.; Monteiro, P.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Heinrich, M.; Barnes, J.; Prieto, J.M.; Gibbons, S.; Williamson, E.M. Complementary/alternative or “integrative” therapies involving use of plant substances. In Fundamentals of Pharmacognosy and Phytotherapy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–345. [Google Scholar]
- Sourmaghi, M.H.S.; Kiaee, G.; Golfakhrabadi, F.; Jamalifar, H.; Khanavi, M. Comparison of essential oil composition and antimicrobial activity of Coriandrum sativum L. extracted by hydrodistillation and microwave-assisted hydrodistillation. J. Food Sci. Technol. 2015, 52, 2452–2457. [Google Scholar] [CrossRef]
- Zekri, N.; Elazzouzi, H.; Drioche, A.; Satrallah, A.; El Belghiti, M.A.; Zair, T. Effect of geographic locations on chemical composition of M. spicata L. essential oils from Moroccan Middle-Atlas. Der Pharm. Lett. 2016, 8, 146–150. [Google Scholar]
- Jaramillo-Colorado, B.; Julio-Torres, J.; Duarte-Restrepo, E.; Gonzalez-Coloma, A.; Julio-Torres, L.F. Comparative study of volatile composition and biological activities of essential oil from Colombian Piper marginatum Jacq. Bol. Latinoam. Caribe Plantas Med. Aromat. 2015, 14, 343–354. [Google Scholar]
- Karousou, R.; Hanlidou, E.; Kokkni, S. The Sage plants in Greece: Distribution and intraspecific variation. In Sage. The genus Salvia; Kintzios, S.E., Ed.; Taylor & Francis: London, UK, 2005; pp. 1–281. [Google Scholar]
- Carvalho Filho, J.L.S.; Blank, A.F.; Alves, P.B.; Ehlert, P.A.D.; Melo, A.S.; Cavalcanti, S.C.H.; Arrigoni-Blank, M.d.F.; Silva-Mann, R. Influence of the harvesting time, temperature and drying period on basil (Ocimum basilicum L.) essential oil. Rev. Bras. Farmacogn. 2006, 16, 24–30. [Google Scholar] [CrossRef]
- Inan, M.; Kirpik, M.; Kaya, D.A.; Kirici, S. Effect of harvest time on essential oil composition of Thymbra spicata L. growing in flora of Adiyaman. Adv. Environ. Biol. 2011, 5, 356–358. [Google Scholar]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef]
- Degani, A.V.; Dudai, N.; Bechar, A.; Vaknin, Y. Shade effects on leaf production and essential oil content and composition of the novel herb Eucalyptus citriodora Hook. J. Essent. Oil Bear. Plants 2016, 19, 410–420. [Google Scholar] [CrossRef]
- Ibrahim, J.A.; Mustapha, B.; Ogah, J.I.; Egharevba, H.O. Comparative pharmacognostic and chemical analyses of Eucalyptus camaldulensis Dehnh and Eucalyptus citriodora (Hook). J. Chem. Soc. Niger. 2018, 43, 560–568. [Google Scholar]
- Smigielski, K.; Prusinowska, R.; Stobiecka, A.; Kunicka-Styczyñska, A.; Gruska, R. Biological properties and chemical composition of essential oils from flowers and aerial parts of lavender (Lavandula angustifolia). J. Essent. Oil Bear. Plants 2018, 21, 1303–1314. [Google Scholar] [CrossRef]
- Danh, L.T.; Han, L.N.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioprocess Technol. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- Cãlin, J.; Miscã, C.; Gruia, A.T.; Bujancã, G.; Stoin, D. Lavandula angustifolia “Sevtopolis” essential oil: The chemical composition and antimicrobial properties. In Proceedings of the International Multidisciplinary Scientific GeoConference. Surveying Geology & Mining Ecology Management (SGEM), Albena, Bulgaria, 29 June–5 July 2017; pp. 281–286. [Google Scholar]
- Wesołowska, A.; Jadczak, P.; Kulpa, D.; Przewodowski, W. Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils from AgNPs and AuNPs elicited Lavandula angustifolia in vitro cultures. Molecules 2019, 24, 606. [Google Scholar] [CrossRef]
- Prusinowska, R.; Smigielski, K.; Stobiecka, A.; Kunicka-Styczyńska, A. Hydrolates from lavender (Lavandula angustifolia)–Their chemical composition as well as aromatic, antimicrobial and antioxidant properties. Nat. Prod. Res. 2016, 30, 386–393. [Google Scholar] [CrossRef]
- Canale, A.; Conti, F.; Mehlhorn, H.; Nicoletti, M.; Cianfaglione, K.; Ciaschetti, G.; Maggi, F.; Benelli, G.; Pavela, R.; Senthil-Nathan, S. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar]
- Yang, X.; Zhao, H.T.; Wang, J.; Meng, Q.; Zhang, H.; Yao, L.; Zhang, Y.C.; Dong, A.J.; Ma, Y.; Wang, Z.Y. Chemical composition and antioxidant activity of essential oil of pine cones of Pinus armandii from the Southwest region of China. J. Med. Plants Res. 2010, 4, 1668–1672. [Google Scholar]
- Tümen, I.; Hafizogen, H.; Kilic, A.; Dönmez, I.E.; Sivrikaya, H.; Reunamen, M. Yield and constituents of essential oils from cones of Pinaceae spp. native growing in Turkey. Molecules 2010, 15, 5797–5806. [Google Scholar] [CrossRef]
- Shao, P.; Ma, H.; Qiu, Q.; Jing, W. Physical stability of R-(+)-Limonene emulsions stabilized by Ulva fasciata algae polysaccharide. Int. J. Biol. Macromol. 2016, 92, 926–934. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, H.; Niu, B.; Jiang, L. Antibacterial activities of R-(+)-Limonene emulsion stabilized by Ulva fasciata polysaccharide for fruit preservation. Int. J. Biol. Macromol. 2018, 111, 1273–1280. [Google Scholar] [CrossRef]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Zafar, I.; Fatima, A.; Khan, S.; Rehman, Z.; Mehmud, S. GC-MS studies of needles essential oil of Pinus roxburghaii and their antimicrobial activity from Pakistan. Electron. J. Environ. Agric. Food Chem. 2010, 9, 468–473. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K.; Arora, K. Phytotoxicity of the volatile monoterpene citronellal against some weeds. Z. Naturforsch 2006, 61c, 334–340. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Vishwakarma, G.S. Phytotoxic potential of essential oil from leaves of Eucalyptus tereticornis against rice (Oryza sativa) and its weeds, Echinochloa crus-galli and Cyperus rotundus. Master’s Thesis, Central University of Punjab, Bathinda, India, 2012. [Google Scholar]
- Khare, P.; Srivastava, S.; Nigam, N.; Singh, A.K.; Singh, S. Impact of essential oils of E. citriodora, O. basilicum and M. arvensis on three different weeds and soil microbial activities. Environ. Technol. Innov. 2019, 14, 1–18. [Google Scholar] [CrossRef]
- Perez, A.; Kogan, M. Glyphosate-resistant Lolium multiflorum in Chilean orchards. Weed Res. 2003, 43, 12–19. [Google Scholar] [CrossRef]
- Rauch, T.A.; Thill, D.C.; Gersdorf, S.A.; Price, W.J. Widespread occurrence of herbicide-resistant Italian Ryegrass (Lolium multiflorum) in Northern Idaho and Eastern Washington. Weed Technol. 2017, 24, 281–288. [Google Scholar] [CrossRef]
- Moss, S.R.; Hull, R.; Perryman, S.A.; Cussans, J.W. Lolium multiflorum: Aspects of herbicide resistance, agro-ecology and effects on crop yield in wheat crops. Asp. Appl. Biol. 2017, 134, 151–160. [Google Scholar]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kaur, S.; Ahuja, N.; Kohli, R.K. β -Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Carbó, E. Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crops Prod. 2015, 76, 515–521. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| RICal | RIRef | Compound | E. citriodora Relative Area (%) | L. angustifolia Relative Area (%) | P. sylvestris Relative Area (%) |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 1.5 ± 0.1 | 7.8 ± 0.1 | 74.4 ± 0.3 | ||
| 924 | 926 | Tricyclene | - | t | 0.1 ± 0.0 |
| 926 | 930 | α-Thujene | t | - | - |
| 939 | 939 | α-Pinene | 0.2 ± 0.0 | 2.5 ± 0.0 | 25.6 ± 0.2 |
| 953 | 954 | Camphene | - | 0.7 ± 0.0 | 6.4 ± 0.1 |
| 977 | 975 | Sabinene | t | 0.3 ± 0.0 | - |
| 985 | 979 | β-Pinene | 0.5 ± 0.0 | 2.4 ± 0.0 | 15.9 ± 0.1 |
| 980 | 987 | 3-p-Menthene | - | - | 0.2 ± 0.0 |
| 998 | 990 | Myrcene | 0.1 ± 0.0 | 0.5 ± 0.0 | 3.5 ± 0.0 |
| 1012 | 1011 | δ-3-Carene | - | - | 0.6 ± 0.0 |
| 1020 | 1017 | α-Terpinene | t | 0.1 ± 0.0 | 2.3 ± 0.0 |
| 1021 | 1024 | p-Cymene | t | 0.5 ± 0.0 | 0.9 ± 0.0 |
| 1028 | 1029 | Limonene | t | - | 18.5 ± 0.2 |
| 1043 | 1037 | cis-Ocimene | - | 0.1 ± 0.1 | - |
| 1053 | 1050 | trans-β-Ocimene | 0.1 ± 0.0 | 0.1 ± 0.0 | - |
| 1056 | 1059 | γ-Terpinene | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 |
| 1090 | 1088 | Terpinolene | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Oxygenated monoterpenes | 94.7 ± 1.2 | 85.5 ± 0.1 | 23.4 ± 0.3 | ||
| 1029 | 1031 | 1,8-Cineole | 0.3 ± 0.0 | 26.5 ± 0.0 | 2.1 ± 0.2 |
| 1051 | 1056 | Bergamal | 0.1 ± 0.0 | - | - |
| 1070 | 1070 | cis-Sabinene Hydrate | - | 0.2 ± 0.0 | - |
| 1076 | 1072 | cis-Linalool Oxide | - | 0.1 ± 0.0 | - |
| 1095 | 1096 | Linalool | 0.1 ± 0.0 | 38.7 ± 0.1 | t |
| 1098 | 1099 | α-Pinene Oxide | - | - | 0.1 ± 0.0 |
| 1104 | 1108 | cis-Rose Oxide | 0.1 ± 0.0 | - | - |
| 1122 | 1125 | trans-Rose Oxide | t | - | - |
| 1129 | Plinol C | - | 0.4 ± 0.1 | - | |
| 1144 | 1146 | Camphor | - | 14.2 ± 0.1 | 0.5 ± 0.0 |
| 1150 | 1149 | Isopulegol | 4.3 ± 1.1 | - | - |
| 1154 | 1153 | Citronellal | 88.0 ± 0.8 | - | - |
| 1158 | 1159 | iso-Isopulegol | 0.5 ± 0.1 | - | - |
| 1159 | 1160 | Isoborneol | - | 0.4 ± 0.0 | - |
| 1168 | 1166 | δ-Terpineol | - | 0.3 ± 0.0 | - |
| 1170 | 1169 | Borneol | - | 1.3 ± 0.0 | - |
| 1179 | 1177 | Terpinen-4-ol | - | 0.3 ± 0.0 | t |
| 1184 | 1182 | p-Cymen-8-ol | - | 0.1 ± 0.0 | - |
| 1187 | 1185 | Cryptone | - | t | - |
| 1188 | 1188 | α-Terpineol | - | 1.6 ± 0.0 | 0.1 ± 0.0 |
| 1196 | 1195 | Myrtenal | - | 0.1 ± 0.0 | - |
| 1197 | 1199 | γ-Terpineol | - | 0.2 ± 0.0 | - |
| 1212 | 1220 | α-Fenchyl Acetate | - | - | 0.1 ± 0.0 |
| 1231 | 1229 | Nerol | - | 0.1 ± 0.0 | - |
| 1256 | 1252 | Piperitone | - | t | - |
| 1258 | 1252 | Geraniol | - | 0.2 ± 0.0 | - |
| 1260 | 1257 | Linalool Acetate | - | 0.5 ± 0.0 | t |
| 1287 | 1288 | Bornyl Acetate | - | 0.1 ± 0.0 | 17.9 ± 0.0 |
| 1311 | 1313 | Citronellic Acid | 0.1 ± 0.0 | - | - |
| 1325 | β-Terpinyl Acetate | - | - | 0.1 ± 0.0 | |
| 1345 | 1349 | α-Terpinyl Acetate | - | - | 2.6 ± 0.0 |
| 1348 | 1352 | Citronellyl Acetate | 1.3 ± 0.1 | - | - |
| 1368 | 1361 | Neryl Acetate | - | 0.2 ± 0.0 | - |
| 1468 | 1468 | Linalool Isovalerate | - | 0.1 ± 0.0 | - |
| 1512 | 1511 | Lavandulyl 2-Methyl Butanoate | - | 0.1 ± 0.0 | - |
| Sesquiterpene hydrocarbons | 2.1 ± 0.2 | 3.3 ± 0.0 | 0.7 ± 0.0 | ||
| 1330 | 1338 | δ-Elemene | - | - | t |
| 1377 | 1376 | α-Copaene | - | t | - |
| 1381 | 1381 | Daucene | - | t | - |
| 1383 | 1388 | β-Bourbonene | - | 0.1 ± 0.0 | - |
| 1385 | 1390 | β-Elemene | - | - | t |
| 1391 | 1391 | 7-epi-Sesquithujene | - | 0.1 ± 0.0 | - |
| 1403 | 1405 | Sesquithujene | - | 0.1 ± 0.0 | - |
| 1407 | 1407 | Longifolene | - | - | 0.1 ± 0.0 |
| 1409 | 1409 | α-Gurjunene | - | 0.1 ± 0.0 | - |
| 1410 | 1411 | α-Cedrene | - | - | 0.1 ± 0.0 |
| 1420 | 1419 | β-Caryophyllene | 2.0 ± 0.2 | 1.8 ± 0.0 | 0.4 ± 0.0 |
| 1427 | 1434 | α-trans-Bergamotene | - | 0.1 ± 0.0 | - |
| 1435 | 1436 | γ-Elemene | - | - | t |
| 1454 | 1454 | α-Humulene | - | 0.1 ± 0.0 | t |
| 1460 | 1456 | trans-β-Farnesene | - | 0.2 ± 0.0 | - |
| 1470 | 1472 | Dauca-5,8-diene | - | t | - |
| 1481 | 1479 | γ-Muurolene | - | 0.3 ± 0.0 | - |
| 1495 | 1500 | Bicyclogermacrene | 0.1 ± 0.0 | - | - |
| 1500 | 1500 | α-Muurolene | - | - | t |
| 1510 | 1505 | β-Bisabolene | - | 0.2 ± 0.0 | - |
| 1514 | 1513 | γ-Cadinene | - | 0.2 ± 0.0 | t |
| 1524 | 1522 | trans-Calamenene | - | t | - |
| 1525 | 1523 | δ-Cadinene | - | t | 0.1 ± 0.0 |
| Germacrene B | - | - | t | ||
| Oxygenated sesquiterpenes | t | 0.3 ± 0.0 | 0.3 ± 0.0 | ||
| 1582 | 1583 | Caryophyllene Oxide | t | 0.2 ± 0.0 | t |
| 1599 | 1600 | Cedrol | - | - | 0.1 ± 0.0 |
| 1641 | 1640 | epi-α-Cadinol | - | 0.1 ± 0.0 | - |
| 1684 | 1685 | α-Bisabolol | - | t | - |
| Oxygenated Diterpenes | - | - | 0.1 ± 0.0 | ||
| 1985 | 1987 | Manool Oxide | - | - | 0.1 ± 0.0 |
| Aromatic compounds | 0.1 ± 0.0 | t | 0.3 ± 0.0 | ||
| 1247 | 1250 | p-Anis Aldehyde | - | - | 0.3 ± 0.0 |
| 1351 | 1359 | Eugenol | 0.1 ± 0.0 | - | - |
| 1434 | 1434 | Coumarin | - | t | - |
| Others | 0.1 ± 0.0 | 0.5 ± 0.1 | - | ||
| 868 | 870 | n-Hexanol | - | t | - |
| 910 | Isobutyl Isobutyrate | 0.1 ± 0.0 | - | - | |
| 983 | 979 | 1-Octen-3-ol | - | t | - |
| 1008 | Isoamyl Isobutyrate | t | - | - | |
| 1194 | 1192 | Hexyl Butanoate | - | 0.1 ± 0.0 | - |
| 1234 | 1332 | Hexyl Tiglate | - | 0.1 ± 0.0 | - |
| 1244 | 1244 | Hexyl Isovalerate | - | 0.3 ± 0.0 | - |
| Total | 98.6 ± 1.2 | 97.6 ± 0.2 | 99.1 ± 0.0 | ||
| Seed Germination (% ± S.E.) | |||||
|---|---|---|---|---|---|
| * Dose | E. citriodora essential oil | ||||
| P. oleracea | L. multiflorum | E. crus-galli | Tomato | Cucumber | |
| Control | 74.0 ± 4.6 a | 65.0 ± 6.9 a | 69.0 ± 2.9 a | 71.0 ± 2.5 a | 99.0 ± 1.0 a |
| 0.125 | 80.0 ± 2.2 a | 67.0 ± 4.4 a | 74.0 ± 3.7 a | 71.0 ± 4.3 a | 98.0 ± 1.2 a |
| 0.25 | 76.0 ± 2.9 a | 52.0 ± 2.0 a | 72.0 ± 2.6 a | 73.0 ± 3.4 a | 95.0 ± 2.2 a |
| 0.5 | 74.0 ± 4.3 a | 58.0 ± 2.6 a | 61.0 ± 4.6 a | 61.0 ± 3.7 a | 97.0 ± 1.2 a |
| 1 | 81.0 ± 6.2 a | 57.0 ± 7.2 a | 72.0 ± 3.7 a | 25.0 ± 11.3 b | 96.0 ± 1.8 a |
| Dose | L. angustifolia essential oil | ||||
| Control | 74.0 ± 3.7 a | 65.0 ± 6.9 a | 71.0 ± 4.3 a | 71.0 ± 2.5 a | 99.0 ± 1.0 a |
| 0.125 | 69.0 ± 5.3 a | 65.0 ± 3.2 a | 71.0 ± 2.8 a | 73.0 ± 4.4 a | 97.0 ± 1.2 a |
| 0.25 | 67.0 ± 2.0 a | 50.0 ± 2.7 a,b | 72.0 ± 2.6 a | 58.0 ± 4.1 a,b | 98.0 ± 2.0 a |
| 0.5 | 66.0 ± 5.8 a | 36.0 ± 8.4 b,c | 72.0 ± 3.4 a | 41.0 ± 13.2 b,c | 97.0 ± 1.2 a |
| 1 | 69.0 ± 3.7 a | 24.0 ± 7.0 c | 58.0 ± 2.6 b | 22.005.8 c | 94.0 ± 1.9 a |
| Dose | P. sylvestris essential oil | ||||
| Control | 75.0 ± 7.1 a | 67.0 ± 2.0 a | 74.0 ± 3.3 a | 68.0 ± 3.4 a | 100.0 ± 0.0 a |
| 0.125 | 74.0 ± 3.7 a | 65.0 ± 8.8 a | 69.0 ± 7.0 a | 67.0 ± 4.4 a | 94.0 ± 2.9 a,b |
| 0.25 | 71.0 ± 2.9 a | 65.0 ± 5.0 a | 74.0 ± 1.9 a | 67.0 ± 4.1 a | 94.0 ± 1.9 a,b |
| 0.5 | 71.0 ± 1.9 a | 58.0 ± 5.2 a | 74.0 ± 4.6 a | 66.0 ± 3.7 a | 95.0 ± 1.6 a,b |
| 1 | 68.0 ± 2.6 a | 51.0 ± 12.8 a | 75.0 ± 5.0 a | 64.0 ± 3.7 a | 90.0 ± 2.3 b |
| Concentration (µL/mL) | E. citriodora | ||
| Germination | Hypocotyl | Radicle | |
| Control | 91.0 ± 3.3 a | 2.5 ± 0.2 a | 3.1 ± 0.3 a |
| 0.125 | 72.00 ± 6.8 a | 1.4 ± 0.3 b | 2.5 ± 0.4 a |
| 0.25 | 68.0 ± 9.0 a | 1.4 ± 0.3 b | 2.5 ± 0.4 a |
| 0.5 | 67.003.4 a | 1.3 ± 0.2 b | 2.5 ± 0.3 a |
| 1 | 66.0 ± 4.7 b | 0.4 ± 0.1 c | 1.0 ± 0.3 b |
| Concentration (µL/mL) | L. angustifolia | ||
| Germination | Hypocotyl | Radicle | |
| Control | 91.0 ± 3.3 a | 2.5 ± 0.3 a | 3.1 ± 0.3 a |
| 0.125 | 81.0 ± 4.0 a | 2.6 ± 0.4 a | 2.8 ± 0.3 a,b |
| 0.25 | 81.0 ± 2.9 a | 2.6 ± 0.2 a | 2.9 ± 0.3 a,b |
| 0.5 | 78.0 ± 3.7 a | 1.8 ± 0.1 a,b | 2.4 ± 0.2 a,b |
| 1 | 64.0 ± 3.7 b | 1.2 ± 0.2 b | 2.0 ± 0.1 b |
| * Dose | Control | 0.125 | 0.25 | 0.5 | 1 | ||
|---|---|---|---|---|---|---|---|
| EC | TO | Hyp | 7.3 ± 1.4 a | 6.8 ± 1.8 a | 5.0 ± 1.2 a,b | 2.7 ± 0.5 a,b | 0.8 ± 0.4 b |
| Rad | 16.7 ± 1.5 a | 14.1 ± 1.7 a | 15.1 ± 1.5 a | 6.3 ± 1.3 b | 3.4 ± 1.7 b | ||
| CU | Hyp | 8.4 ± 0.1 a | 8.3 ± 0.4 a | 8.4 ± 0.2 a | 8.4 ± 0.1 a | 8.5 ± 0.9 a | |
| Rad | 23.1 ± 1.5 a | 20.2 ± 0.5 a | 15.3 ± 0.5 b | 15.1 ± 0.9 b | 13.3 ± 0.4 b | ||
| LA | TO | Hyp | 7.3 ± 1.4 a | 7.2 ± 0.7 a | 4.9 ± 0.9 a,b | 2.1 ± 0.8 b,c | 0.5 ± 0.3 c |
| Rad | 16.7 ± 1.5 a | 16.4 ± 0.9 a | 11.6 ± 1.1 a,b | 7.3 ± 2.1 b,c | 2.8 ± 2.0 c | ||
| CU | Hyp | 8.4 ± 0.1 a | 8.3 ± 1.0 a | 8.1 ± 0.9 a | 8.3 ± 0.9 a | 8.3 ± 0.04 a | |
| Rad | 23.1 ± 1.5 a | 18.9 ± 0.5 b | 17.1 ± 0.5 b,c | 15.6 ± 1.0 b,c | 14.4 ± 0.7 c | ||
| PS | TO | Hyp | 12.6 ± 1.6 a | 3.8 ± 1.2 b | 4.0 ± 0.7 b | 3.2 ± 0.7 b | 3.5 ± 0.3 b |
| Rad | 18.1 ± 1.0 a | 9.4 ± 1.0 b | 10.6 ± 0.5 b | 6.8 ± 1.7 b | 6.7 ± 0.4 b | ||
| CU | Hyp | 8.5 ± 0.9 a | 8.6 ± 0.2 a | 8.4 ± 0.3 a | 8.4 ± 0.8 a | 7.7 ± 0.9 a | |
| Rad | 21.2 ± 1.0 a | 17.8 ± 0.6 a,b | 16.3 ± 1.1 b | 16.5 ± 0.8 b | 15.2 ± 0.8 b | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. https://doi.org/10.3390/molecules24152847
Ibáñez MD, Blázquez MA. Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules. 2019; 24(15):2847. https://doi.org/10.3390/molecules24152847
Chicago/Turabian StyleIbáñez, María Dolores, and María Amparo Blázquez. 2019. "Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species" Molecules 24, no. 15: 2847. https://doi.org/10.3390/molecules24152847
APA StyleIbáñez, M. D., & Blázquez, M. A. (2019). Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules, 24(15), 2847. https://doi.org/10.3390/molecules24152847