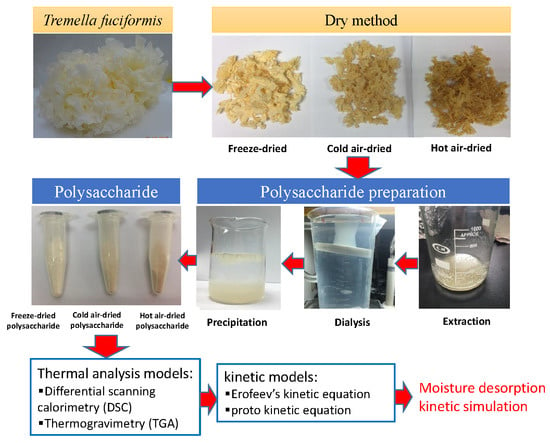
Differences in the Moisture Capacity and Thermal Stability of Tremella fuciformis Polysaccharides Obtained by Various Drying Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Characteristics of Various Drying Processes for T. fuciformis Polysaccharides
2.2. Tremella fuciformis Polysaccharides Moisture Desorption and Thermal Decomposition Properties
2.3. Thermal Mass Loss of T. fuciformis Polysaccharides
2.4. Moisture Content Stability and Characteristics Obtained by Desorption Kinetic Simulation
3. Materials and Methods
3.1. T. fuciformis Samples and Drying Methods
3.2. Dried Products of T. fuciformis Color Measurement
3.3. Preparation of T. fuciformis Polysaccharides
3.4. T. fuciformis Polysaccharide Molecular Distribution Analysis
3.5. T. fuciformis Polysaccharide Moisture Content Measurement
3.6. Tremella fuciformis Polysaccharide DSC Tests
3.7. T. fuciformis Polysaccharide TGA Analyses
3.8. T. fuciformis Polysaccharide Moisture Capacity Kinetic Simulation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reshetnikov, S.V.; Wasser, S.P.; Duckman, I.; Tsukor, K. Medicinal value of the genus Tremella Pers. (Heterobasidiomycetes). Int. J. Med. Mushrooms 2000, 2, 169–193. [Google Scholar] [CrossRef]
- Kiho, T.; Tsujimura, Y.; Sakushima, M.; Usui, S.; Ukai, S. Polysaccharides in fungi. XXXIII. hypoglycemic activity of an acidic polysaccharide (AC) from Tremella fuciformis. Yakugaku Zasshi 1994, 114, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Zhang, L.; Wang, M. Polyethylene glycol-based ultrasound-assisted extraction and ultrafiltration separation of polysaccharides from Tremella fuciformis (snow fungus). Food Bioprod. Process. 2016, 100, 464–468. [Google Scholar] [CrossRef]
- De Baets, S.; Vandamme, E.J. Extracellular Tremella polysaccharides: structure, properties and applications. Biotechnol. Lett. 2001, 23, 1361–1366. [Google Scholar] [CrossRef]
- Kiho, T.; Kobayashi, T.; Morimoto, H.; Usui, S.; Ukai, S.; Hirano, K.; Aizawa, K.; Inakuma, T. Structural features of an anti-diabetic polysaccharide (TAP) from Tremella aurantia. Chem. Pharm. Bull. 2000, 48, 1793–1795. [Google Scholar] [CrossRef] [PubMed]
- Yui, T.; Ogawa, K.; Kakuta, M.; Misaki, A. Chain conformation of a glucurono-xylo-mannan isolated from fruit body of Tremella fuciformis Berk. J. Carbohyd. Chem. 1995, 14, 255–263. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.G.; Yan, Y.H.; Shan, Y.N.; Kan, J.; Jin, C.H. Structure, physical property and antioxidant activity of catechin grafted Tremella fuciformis polysaccharide. Int. J. Biol. Macromol. 2016, 82, 719–724. [Google Scholar] [CrossRef]
- Tang, B.; Liu, J.; Fan, L.; Li, D.; Chen, X.; Zhou, J.; Li, J. Green preparation of gold nanoparticles with Tremella fuciformis for surface enhanced Raman scattering sensing. Appl. Surf. Sci. 2018, 427, 210–218. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, Q.; Lu, J.; Xu, J.L.; Zhang, H.; Wang, J.H. Physicochemical properties of Tremella fuciformis polysaccharide and its interactions with myofibrillar protein. Bioact. Carbohydr. Dietary Fibre 2017, 11, 18–25. [Google Scholar] [CrossRef]
- Wu., Q.; Zheng, C.; Ning, Z.X.; Yang, B. Modification of low molecular weight polysaccharides from Tremella fuciformis and their antioxidant activity in vitro. Int. J. Mol. Sci. 2007, 8, 670–679. [Google Scholar] [CrossRef]
- Hung, L.; Chang, A.J.; Kuo, X.K.; Sheu, F. Molecular cloning and function characterization of a new macrophage-activating protein from Tremella fuciformis. J. Agric. Food. Chem. 2014, 62, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.W.; Liu, Y.; Xu, Y.; Hong, Y.R.; Liu, Q.; Li, X.L.; Wang, Z.G. Tremella polysaccharides attenuated sepsis through inhibiting abnormal CD4(+) CD25 (high) regulatory T cells in mice. Cell Immunol. 2014, 288, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Optimization of extraction of Tremella fuciformis polysaccharides and its antioxidant and antitumour activities in vitro. Carbohydr. Polym. 2010, 81, 420–424. [Google Scholar] [CrossRef]
- Xu, W.; Shen, X.; Yang, F.; Han, Y.; Li, R.; Xue, D.; Jiang, C. Protective effect of polysaccharides isolated from Tremella fuciformis against radiation-induced damage in mice. J. Radiat. Res. 2012, 53, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Hwang, H.J.; Kim., S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic effects of exopolysaccharides produced by mycelial cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Zhao, M. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int. J. Biol. Macromol. 2015, 72, 526–530. [Google Scholar] [CrossRef]
- Wen, L.; Gao, Q.; Ma, C.W.; Ge, Y.; You, L.; Liu, R.H.; Fu, X.; Liu, D. Effect of polysaccharides from Tremella fuciformis on UV-induced photoaging. J. Funct. Foods 2016, 20, 400–410. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Hwang, B.F.; Wang, Y.H.; Lin, C.P. Moisture desorption and thermal properties of polysaccharide from pulsed light irradiated Flammulina velutipes. J. Therm. Anal. Calorim. 2017, 127, 469–481. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, S.Y.; Yu, H.T.; Lin, C.P. Degradation of polylactic acid by irradiation. J. Polym. Environ. 2018, 26, 122–131. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tsai, S.Y.; Tseng, Y.L.; Lin, C.P. Gamma irradiation for improving functional ingredients and determining the heat treatment conditions of Cordyceps militaris mycelia. J. Therm. Anal. Calorim. 2015, 120, 439–448. [Google Scholar] [CrossRef]
- Thermal Safety Software (TSS), ChemInform Saint-Petersburg (CISP) Ltd.: St. Petersburg, Russia, 2011. Available online: http://www.cisp.spb.ru(accessed on 5 August 2019).
- Hsu, C.L.; Chen, W.; Weng, Y.M.; Tseng, C.Y. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003, 83, 85–92. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |






| Sample | L* Value | a* Value | b* Value | WI Value |
|---|---|---|---|---|
| Freeze dried | 79.33 ± 0.09 a | 7.79 ± 0.06 c | 16.02 ± 0.04 c | 72.71 ± 0.12 a |
| Cold air dried | 72.39 ± 0.17 b | 9.48 ± 0.37 b | 19.86 ± 0.64 b | 64.70 ± 0.72 b |
| Hot air dried | 72.59 ± 1.05 b | 10.23 ± 0.27 a | 22.97 ± 0.19 a | 62.81 ± 0.99 c |
| Sample | Yield (%) | Molecular Weight (Da) | Moisture Content (%) |
|---|---|---|---|
| Freeze dried | 15.35 | 2.06 × 107 | 11.61 |
| Cold air dried | 15.60 | 2.41 × 107 | 13.05 |
| Hot air dried | 16.10 | 2.34 × 107 | 12.65 |
| Sample | Mass (mg) | Heating rate (°C/min) | enTo (°C) | enTp (°C) | enΔH (kJ/kg) | exTo (°C) | exTp (°C) | exΔHd (kJ/kg) |
|---|---|---|---|---|---|---|---|---|
| Freeze dried | 1.12 | 6 | 56.27 | 107.84 | 362.70 | 265.81 | 291.16 | 108.70 |
| 1.13 | 8 | 42.98 | 99.30 | 304.20 | 274.10 | 297.54 | 72.73 | |
| 1.02 | 10 | 42.67 | 95.25 | 299.50 | 282.13 | 303.90 | 60.61 | |
| Cold dried | 1.12 | 6 | 41.67 | 89.42 | 321.90 | 269.13 | 293.19 | 79.98 |
| 1.20 | 8 | 41.59 | 91.27 | 331.30 | 277.11 | 299.23 | 52.31 | |
| 1.06 | 10 | 42.09 | 88.83 | 279.50 | 282.25 | 305.50 | 54.64 | |
| Hot dried | 1.02 | 6 | 41.68 | 88.32 | 253.50 | 273.47 | 292.77 | 35.91 |
| 1.60 | 8 | 43.59 | 94.36 | 350.60 | 274.00 | 296.25 | 79.51 | |
| 1.05 | 10 | 46.47 | 90.99 | 221.20 | 280.79 | 302.75 | 48.55 |
| Sample | heating Rate (°C/min) | Nitrogen | Air | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mass (mg) | mdTp (°C) | tdTp (°C) | Mass Loss (mass%) | Mass (mg) | mdTp (°C) | tdTp (°C) | Mass Loss (mass%) | ||
| Freeze dried | 6 | 6.02 | 57.50 | 288.11 | −81.58 | 6.02 | 56.00 | 274.00 | −92.70 |
| 8 | 6.01 | 59.87 | 290.27 | −80.84 | 6.01 | 60.93 | 279.60 | −94.50 | |
| 10 | 6.00 | 63.50 | 296.00 | −80.37 | 6.00 | 62.00 | 296.00 | −93.28 | |
| Cold dried | 6 | 6.00 | 60.00 | 286.00 | −74.72 | 6.01 | 58.00 | 286.00 | −75.54 |
| 8 | 6.01 | 59.87 | 289.20 | −76.74 | 6.01 | 64.13 | 281.73 | −89.70 | |
| 10 | 6.00 | 65.50 | 294.00 | −76.10 | 6.04 | 68.50 | 284.00 | −88.07 | |
| Hot dried | 6 | 6.01 | 57.50 | 284.00 | −76.79 | 6.01 | 58.00 | 274.00 | −91.83 |
| 8 | 6.02 | 64.13 | 288.13 | −77.60 | 6.02 | 59.87 | 279.60 | −88.37 | |
| 10 | 6.00 | 64.50 | 292.00 | −77.38 | 6.02 | 61.50 | 282.00 | −87.95 | |
| Sample | Heating rates (°C/min) | ||||||
|---|---|---|---|---|---|---|---|
| 6 | 8 | 10 | |||||
| Parameter | Erofeev | Proto | Erofeev | Proto | Erofeev | Proto | |
| Freeze dried | ln(k0)/ln(1/s) | 1.0 × 10−6 | 5.9911 | 1.0 × 10−7 | 5.9900 | 1.0 × 10−9 | 6.2444 |
| Ea(kJ/mol) | 18.2713 | 36.8027 | 16.7371 | 34.8600 | 15.6216 | 34.3314 | |
| n/n1 | 0.3944 | 0.1663 | 0.4008 | 0.1950 | 0.4310 | 0.2038 | |
| n2 | N/A | 1.2276 | N/A | 1.1647 | N/A | 1.1601 | |
| ΔH(kJ/kg) | −412.7941 | −418.0235 | −333.7040 | −336.6741 | −336.2525 | −338.0239 | |
| Cold dried | ln(k0) | 1.0 × 10−12 | 8.8783 | 11.0162 | 0.0605 | 10.9169 | 2.8564 |
| Ea | 18.2685 | 46.0057 | 50.5996 | 16.5046 | 47.6868 | 23.6190 | |
| n/n1 | 0.3951 | 0.0122 | 1.3943 | 0.4948 | 1.5242 | 0.3356 | |
| n2 | N/A | 1.3537 | N/A | 0.8450 | N/A | 1.1172 | |
| ΔH | −412.0653 | −418.8865 | −318.4601 | −307.3842 | −377.7577 | −372.8248 | |
| Hot dried | ln(k0) | 1.5744 | 5.3957 | 1.0 × 10−8 | 4.4862 | 1.0 × 10−16 | 3.9560 |
| Ea | 22.0218 | 33.1946 | 16.3484 | 29.6631 | 15.3977 | 26.4318 | |
| n/n1 | 0.2160 | 0.0758 | 0.4104 | 0.2650 | 0.4332 | 0.4225 | |
| n2 | N/A | 1.1473 | N/A | 1.0986 | N/A | 1.1435 | |
| ΔH | −377.8556 | −381.3110 | −398.3055 | −401.7383 | −280.5788 | −282.0463 | |
| Sample Atmospheres Parameter | Heating rates (°C/min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 | 8 | 10 | ||||||
| Erofeev | Proto | Erofeev | Proto | Erofeev | Proto | |||
| Freeze dried | Nitrogen | ln(k0) (ln(1/s)) | 1.0 × 10−8 | 6.8139 | 1.0 × 10−8 | 7.6747 | 1.0 × 10−10 | 9.7388 |
| Ea (kJ/mol) | 15.2080 | 33.1840 | 11.8237 | 32.7078 | 14.1710 | 40.8807 | ||
| n/n1 | 0.2437 | 0.1900 | 0.3164 | 0.1758 | 0.3261 | 0.1563 | ||
| n2 | N/A | 1.4639 | N/A | 1.3277 | N/A | 1.4953 | ||
| DM (mass%) | −9.8306 | −10.0380 | −10.0056 | −10.1331 | −9.8335 | −9.9987 | ||
| Cold dried | ln(k0) | 18.3377 | 4.7873 | 3.0 × 10−8 | 8.9333 | 3.2 × 10−8 | 1.9764 | |
| Ea | 64.7625 | 28.1377 | 15.7559 | 39.3054 | 14.9599 | 19.7081 | ||
| n/n1 | 2.7817 | 0.2221 | 0.1584 | 0.1354 | 0.2556 | 0.3243 | ||
| n2 | N/A | 1.6567 | N/A | 2.1329 | N/A | 1.4310 | ||
| DM | −10.3558 | −10.1801 | −10.4496 | −11.1291 | −9.4918 | −10.1712 | ||
| Hot dried | ln(k0) | 1.0 × 10−6 | 7.9012 | 1.0 × 10−10 | 8.7524 | 1.0 × 10−8 | 15.5255 | |
| Ea | 15.3605 | 36.3266 | 12.0932 | 36.0103 | 14.4254 | 57.3172 | ||
| n/n1 | 0.2440 | 0.1720 | 0.3100 | 0.1444 | 0.3158 | 2.6 × 10−3 | ||
| n2 | N/A | 1.6172 | N/A | 1.4510 | N/A | 1.9482 | ||
| DM | −9.7767 | −10.0970 | −9.7811 | −9.9547 | −9.6731 | −9.9811 | ||
| Freeze dried | Air | ln(k0) | 1.0 × 10−6 | 9.2593 | 6.3 × 10−8 | 8.2717 | 0.1711 | 7.7847 |
| Ea | 15.0903 | 39.9022 | 14.7013 | 37.2788 | 14.8925 | 35.8396 | ||
| n/n1 | 0.2657 | 0.1387 | 0.2818 | 0.1202 | 0.2851 | 0.1321 | ||
| n2 | N/A | 1.5232 | N/A | 1.3931 | N/A | 1.3813 | ||
| DM | −9.7068 | −9.9269 | −9.8599 | −10.0177 | −9.8607 | −10.0220 | ||
| Cold dried | ln(k0) | 1.0 × 10−5 | 7.1195 | 1.0 × 10−7 | 12.0832 | 2.4 × 10−10 | 6.1735 | |
| Ea | 16.0985 | 34.5655 | 16.7190 | 49.2521 | 15.1980 | 31.6670 | ||
| n/n1 | 0.2016 | 0.2193 | 0.1780 | 0.0317 | 0.2109 | 0.1742 | ||
| n2 | N/A | 1.9456 | N/A | 2.1931 | N/A | 1.7107 | ||
| DM | −9.7935 | −10.4593 | −10.3370 | −10.9423 | −10.1435 | −10.6442 | ||
| Hot dried | ln(k0) | 1.0 × 10−3 | 11.2793 | 1.0 × 10−7 | 10.3135 | 0.3072 | 6.7677 | |
| Ea | 15.3679 | 45.5960 | 14.8336 | 42.8635 | 15.3190 | 32.9951 | ||
| n/n1 | 0.2304 | 0.0735 | 0.2535 | 0.0659 | 0.2716 | 0.1555 | ||
| n2 | N/A | 1.7008 | N/A | 1.5940 | N/A | 1.3461 | ||
| DM | −10.6665 | −10.9321 | −10.2557 | −10.4841 | −10.2999 | −10.4632 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-P.; Tsai, S.-Y. Differences in the Moisture Capacity and Thermal Stability of Tremella fuciformis Polysaccharides Obtained by Various Drying Processes. Molecules 2019, 24, 2856. https://doi.org/10.3390/molecules24152856
Lin C-P, Tsai S-Y. Differences in the Moisture Capacity and Thermal Stability of Tremella fuciformis Polysaccharides Obtained by Various Drying Processes. Molecules. 2019; 24(15):2856. https://doi.org/10.3390/molecules24152856
Chicago/Turabian StyleLin, Chun-Ping, and Shu-Yao Tsai. 2019. "Differences in the Moisture Capacity and Thermal Stability of Tremella fuciformis Polysaccharides Obtained by Various Drying Processes" Molecules 24, no. 15: 2856. https://doi.org/10.3390/molecules24152856
APA StyleLin, C. -P., & Tsai, S. -Y. (2019). Differences in the Moisture Capacity and Thermal Stability of Tremella fuciformis Polysaccharides Obtained by Various Drying Processes. Molecules, 24(15), 2856. https://doi.org/10.3390/molecules24152856