Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent
Abstract
:1. Introduction
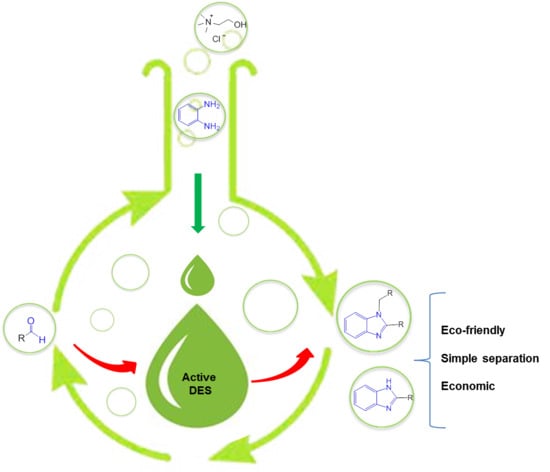
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for DESs Preparation
3.3. General Procedure for the Synthesis of 2-Substituted Benzimidazoles 1a–8a in the DES ChCl:o–PDA (1:1)
3.4. General Procedure for the Synthesis of 1,2-Substituted Benzimidazoles 1b–8b in the DES ChCl:o–PDA (1:1)
3.5. Differential Scanning Analysis (DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alaqeel, S.I. Synthetic approaches to benzimidazoles from o-phenylenediamine: A literature review. J. Saudi Chem. Soc. 2017, 21, 229–237. [Google Scholar] [CrossRef]
- Emerson, G.; Brink, N.G.; Holly, F.W.; Koniuszy, F.; Heyl, D.; Folker, K. Vitamin B12. VIII. Vitamin B12-Like activity of 5,6-dimethylbenzimidazole and tests on related compounds. J. Am. Chem. Soc. 1950, 72, 3084–3085. [Google Scholar] [CrossRef]
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.; Sharma, D.; Kumar, P. Benzimidazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012, 21, 269–283. [Google Scholar] [CrossRef]
- Algul, O.; Karabulut, A.; Canacankatan, N.; Gorur, A.; Sucu, N.; Vezir, O. Apoptotic and anti-angiogenic effects of benzimidazole compounds: Relationship with oxidative stress mediated ischemia/reperfusion injury in rat hind limb. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 267–275. [Google Scholar] [CrossRef]
- Zhu, G.-D.; Gandhi, V.B.; Gong, J.; Thomas, S.; Luo, Y.; Liu, X.; Shi, Y.; Klinghofer, V.; Johnson, E.F.; Frost, D.; et al. Synthesis and SAR of novel, potent and orally bioavailable benzimidazole inhibitors of poly(ADP-ribose) polymerase (PARP) with a quaternary methylene-amino substituent. Bioorg. Med. Chem. Lett. 2008, 18, 3955–3958. [Google Scholar] [CrossRef]
- Ogino, Y.; Ohtake, N.; Nagae, Y.; Matsuda, K.; Moriya, M.; Suga, T.; Ishikawa, M.; Kanesaka, M.; Mitobe, Y.; Ito, J.; et al. Design, syntheses, and structure-activity relationships of novel NPY Y5 receptor antagonists: 2-{3-Oxospiro[isobenzofuran-1(3H),4′-piperidin]-1′-yl} benzimidazole derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 5010–5014. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Rathod, C.P.; Rajurkar, R.M.; Thonte, S.S. Benzimidazole synthesis and biological evaluation: A review. Indo Am. J. Pharm. Res. 2013, 3, 2323–2329. [Google Scholar]
- Thakuria, H.; Das, G. An expeditious one-pot solvent-free synthesis of benzimidazole derivatives. Arkivoc 2008, 15, 321–328. [Google Scholar]
- Rithe, S.R.; Jagtap, R.S.; Ubarhande, S.S. One pot synthesis of substituted benzimidazole derivatives and their characterization. RASAYAN J. Chem. 2015, 8, 213–217. [Google Scholar]
- Saberi, A. Efficient synthesis of Benzimidazoles using zeolite, alumina and silica gel under microwave irradiation. Iran. J. Sci. Technol. 2015, 39, 7–10. [Google Scholar]
- Mobinikhaledi, A.; Hamta, A.; Kalhor, M.; Shariatzadeh, M. Simple Synthesis and Biological Evaluation of Some Benzimidazoles Using Sodium Hexafluroaluminate, Na3 AlF6, as an Efficient Catalyst. Iran. J. Pharm. Res. 2014, 13, 95–101. [Google Scholar] [PubMed]
- Birajdar, S.S.; Hatnapure, G.D.; Keche, A.P.; Kamble, V.M. Synthesis of 2-substituted-1 H-benzo[d]imidazoles through oxidative cyclization of O-phenylenediamine and substituted aldehydes using dioxanedibromide. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 487–493. [Google Scholar]
- Srinivasulu, R.; Kumar, K.R.; Satyanarayana, P.V.V. Facile and efficient method for synthesis of benzimidazole derivatives catalyzed by zinc triflate. Green Sustain. Chem. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- Sehyun, P.; Jaehun, J.; Eun, J.C. Visible-Light-Promoted Synthesis of Benzimidazoles. J. Org. Chem. 2014, 2014, 4148–4154. [Google Scholar]
- Vishvanath, D.P.; Ketan, P.P. Synthesis of Benzimidazole and Benzoxazole Derivatives Catalyzed by Nickel Acetate as Organometallic Catalyst. Int. J. ChemTech Res. 2014, 8, 457–465. [Google Scholar]
- Wan, J.P.; Gan, S.F.; Wu, J.M.; Pan, Y. Water mediated chemoselective synthesis of 1,2-disubstituted benzimidazoles using o-phenylenediamine and the extended synthesis of quinoxalines. Green Chem. 2009, 11, 1633–1637. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Aniket, P.; Shantanu, D.S.; Anagha, O.B.; Ajinkya, P.S. Iodine catalyzed convenient synthesis of 2-aryl-1-arylmethyl-1 H-benzimidazoles in aqueous media. Int. J. ChemTech Res. 2015, 8, 496–500. [Google Scholar]
- Herrera Cano, N.; Uranga, J.G.; Nardi, M.; Procopio, A.; Wunderlin, D.A.; Santiago, A.N. Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. The role of the Er(OTf)3 catalyst in the reaction selectivity. Beilstein J. Org. Chem. 2016, 12, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Hussey, C.L. Room temperature haloaluminate ionic liquids. Novel solvents for transition metal solution chemistry. Pure Appl. Chem. 1988, 60, 1763–1772. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Kubisa, P. Potential for the use of ionic liquids in polymer chemistry. Int. Polym. Sci. Technol. 2006, 33, 445–450. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.; Keim, W.; Wasserscheid, P. Linear dimerisation of but-1-ene in biphasic mode using buffered chloroaluminate ionic liquid solvents. Chem. Commun. 1999, 4, 337–338. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-microbial activities of ionic liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Sapkal, S.B.; Shelke, K.F.; Sonar, S.S.; Shingate, B.B.; Shingare, M.S. Acidic ionic liquid catalyzed environmentally friendly synthesis of benzimidazole derivatives. Bull. Catal. Soc. India 2009, 2, 78–83. [Google Scholar]
- Ranke, J.; Stolte, S.; Stormann, R.; Arning, J.; Jastorff, B. Design of sustainable chemical products–the example of ionic liquids. Chem. Rev. 2007, 107, 2183–2206. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Clark, J.H.; Tavener, S.J. Alternative solvents: shades of green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Tagarelli, A.; Sindona, G. Simple and efficient MW-assisted cleavage of acetals and ketals in pure water. Tetrahedron Lett. 2007, 48, 8623–8627. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Rosati, O. Highly efficient and versatile chemoselective addition of amines to epoxides in water catalyzed by erbium(III) triflate. Tetrahedron Lett. 2008, 49, 2289–2293. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Paonessa, R. An eco-sustainable erbium(III) triflate catalyzed formation and cleavage of tert -butyl ethers. Synthesis 2011, 1, 73–78. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Paonessa, R.; Nardi, M.; Procopio, A. Catalyst-free tosylation of lipophilic alcohols in water. RSC Adv. 2013, 3, 2548–2552. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Olivito, F.; Costanzo, P.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. Efficient synthesis of organic thioacetate in water. Org. Biomol. Chem. 2018, 16, 7753–7759. [Google Scholar] [CrossRef]
- Leitner, W.; Poliakoff, M. Supercritical fluids in green chemistry. Green Chem. 2008, 10, 730. [Google Scholar]
- Carlès, P. A brief review of the thermophysical properties of supercritical fluids. J. Supercrit. Fluid. 2010, 53, 2–11. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Barattucci, A.; Bonaccorsi, P.; Leggio, A.; Minuti, L.; Romio, E.; Temperini, A.; Siciliano, C. Deprotection/reprotection of the amino group in α-amino acids and peptides. A one-pot procedure in [Bmim][BF4] ionic liquid. RSC Adv. 2014, 4, 2678–2686. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-López, D.; Russo, B.; Algieri, V. Efficient organocatalyst supported on a simple ionic liquid as a recoverable system for the asymmetric diels-alder reaction in the presence of water. Chem. Cat. Chem. 2015, 7, 830–835. [Google Scholar]
- Belsito, E.L.; De Marco, R.; Di Gioia, M.L.; Liguori, A.; Perri, F.; Viscomi, M.C. N-(4-nitrophenylsulfonyl)- and N-(fluorenylmethoxycarbonyl)-N-ethyl amino acid methyl esters. Eur. J. Org. Chem. 2010, 22, 4245–4252. [Google Scholar] [CrossRef]
- De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A.; Perri, F.; Siciliano, C.; Viscomi, M.C. A new non-natural arginine-like amino acid derivative with a sulfamoyl group in the side-chain. Amino Acids 2010, 38, 691–700. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Gagliardi, A.; Leggio, A.; Leotta, V.; Romio, E.; Liguori, A. N-Urethane protection of amines and amino acids in an ionic liquid. RSC Adv. 2015, 5, 63407–63420. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- Leggio, A.; Di Gioia, M.L.; Perri, F.; Liguori, A. N-Nosyl-α-amino acids in solution phase peptide synthesis. Tetrahedron 2007, 63, 8164–8173. [Google Scholar] [CrossRef]
- De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-functionalized 1,2,3-triazoles using ionic liquid/iron(III) chloride as an efficient and reusable homogeneous catalyst. Catalysts 2018, 8, 364–376. [Google Scholar]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) chloride in ethyl lactate as a smart ecofriendly system for efficient and rapid stereoselective synthesis of trans-4,5-diaminocyclopent-2-enones. ACS Sustain Chem. Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Nardi, M.; Cano, N.H.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/Ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable deep eutectic solvent for selective and scalable conversion of furfural into cyclopentenone derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 7, 70–71. [Google Scholar] [CrossRef]
- Maugeri, Z.; Dominguez de Maria, P. Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: Levulinic acid and sugar-based polyols. RSC Adv. 2012, 2, 421–425. [Google Scholar] [CrossRef]
- Nagata, K.; Itoh, T.; Ishikawa, H.; Ohsawa, A. Synthesis of 2-substituted benzimidazoles by reaction of o-phenylenediamine with aldehydes in the presence of Sc (OTf). Heterocycles 2003, 61, 93–96. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef]
- Adeyemi, I.; Abu-Zahra, M.R.M.; Alnashef, I. Novel green solvents for CO2 capture. Energy Procedia 2017, 114, 2552–2560. [Google Scholar] [CrossRef]
- Piotr, S.; Tadeusz, S. New aromatic diamine-based deep eutectic solvents designed for epoxy resin curing. Polimery 2018, 63, 453–458. [Google Scholar]
- Abbot, A.; Capper, G.; Davies, L.; Rasheed, K. Ionic liquid analogues formed from hydrated metal salts. Chem. Eur. J. 2004, 10, 3769–3774. [Google Scholar] [CrossRef] [PubMed]
- Abbot, A.; Capper, G.; Davies, L.; Rasheed, K.; Archer, J.; John, C. Electrodeposition of chromium black from ionic liquids. Trans. Inst. Met. Finish 2004, 82, 14–17. [Google Scholar] [CrossRef]
- Abbott, A.; Capper, G.; McKenzie, K.J.; Ryder, K.S. Electrodeposition of zinc–tin alloys from deep eutectic solvents based on choline chloride. J. Electroanal. Chem. 2007, 599, 288–294. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, K.; Zhu, Y.; Zhu, R.; Ye, F.; Song, N.; Xu, Y. Physicochemical properties of deep eutectic solvents formed by choline chloride and phenolic compounds at T = (293.15 to 333.15) K: The influence of electronic effect of substitution group. J. Mol. Liq. 2017, 232, 182–187. [Google Scholar] [CrossRef]
- Kumar, D.; Kommi, D.N.; Chebolu, R.; Garg, S.K.; Kumar, R.; Chakraborti, A.K. Selectivity control during the solid supported protic acids catalysed synthesis of 1,2-disubstituted benzimidazoles and mechanistic insight to rationalize selectivity. RSC Adv. 2013, 3, 91–98. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 0-19-850234-6. [Google Scholar]
Sample Availability: Samples of the compounds 1a–8a, 1b–6b are available from the authors. |





| Entry | Solvent | Molar Ratio o-PDA:Benzaldehyde | T (°C) | Time (min) | Yield (%) 3 a:b |
|---|---|---|---|---|---|
| 1 1 | ChCl:urea (1:2) | 1:1 | 60 | 15 | 67:33 |
| 2 2 | ChCl:urea (1:2) | 1:2 | 60 | 15 | 30:70 |
| 3 1 | ChCl:urea (1:2) | 1:1 | 80 | 10 | 88:12 |
| 4 2 | ChCl:urea (1:2) | 1:2 | 80 | 10 | 13:87 |
| ChCl | HBD | Molar Ratio | Tf (°C) | Tm HBD (°C) | Δ (°C) | Appearance |
|---|---|---|---|---|---|---|
 |  | 1:1 | 32 | 102 | 70 | Light yellow liquid that tends to become greenish. |

| Entry | R | Product | Yields b (%) | |
|---|---|---|---|---|
| 1 | Ph |  | 1a | 95 (93) c |
| 2 | p-MePh |  | 2a | 97 |
| 3 | p-MeOPh |  | 3a | 92 |
| 4 | CH3CH2 |  | 4a | 95 |
| 5 | CH3 |  | 5a | 96 |
| 6 | CH2Ph |  | 6a | 91 |
| 7 | p-ClPh |  | 7a | 90 |
| 8 | p-NO2Ph |  | 8a | 89 |

| Entry | R | Product | Yields b (%) | |
|---|---|---|---|---|
| 1 | Ph |  | 1b | 97 |
| 2 | p-MePh |  | 2b | 98 |
| 3 | p-MeOPh |  | 3b | 93 |
| 4 | CH3CH2 |  | 4b | 90 |
| 5 | CH3 |  | 5b | 91 |
| 6 | CH2Ph |  | 6b | 91 |
| 7 c | p-ClPh |  | 7b | 0 |
| 8 c | p-NO2Ph |  | 8b | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gioia, M.L.; Cassano, R.; Costanzo, P.; Herrera Cano, N.; Maiuolo, L.; Nardi, M.; Nicoletta, F.P.; Oliverio, M.; Procopio, A. Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Molecules 2019, 24, 2885. https://doi.org/10.3390/molecules24162885
Di Gioia ML, Cassano R, Costanzo P, Herrera Cano N, Maiuolo L, Nardi M, Nicoletta FP, Oliverio M, Procopio A. Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Molecules. 2019; 24(16):2885. https://doi.org/10.3390/molecules24162885
Chicago/Turabian StyleDi Gioia, Maria Luisa, Roberta Cassano, Paola Costanzo, Natividad Herrera Cano, Loredana Maiuolo, Monica Nardi, Fiore Pasquale Nicoletta, Manuela Oliverio, and Antonio Procopio. 2019. "Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent" Molecules 24, no. 16: 2885. https://doi.org/10.3390/molecules24162885
APA StyleDi Gioia, M. L., Cassano, R., Costanzo, P., Herrera Cano, N., Maiuolo, L., Nardi, M., Nicoletta, F. P., Oliverio, M., & Procopio, A. (2019). Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Molecules, 24(16), 2885. https://doi.org/10.3390/molecules24162885