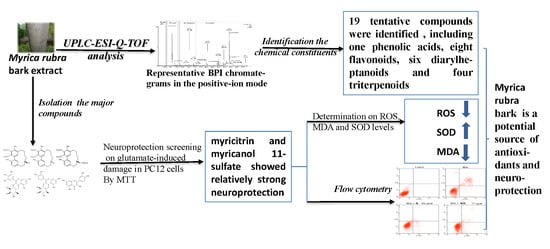
Study on the Material Basis of Neuroprotection of Myrica rubra Bark
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of the Chemical Constituents of MRB by UPLC-PDA and UPLC-Q-TOF-MS Analysis
2.1.1. Phenolic Acid (Peak 1)
2.1.2. Flavonoids (Peaks 2–9)
2.1.3. Diarylheptanoids (Peaks 10–15)
2.1.4. Triterpenoids (Peaks 16–19)
2.2. Neuroprotection of Six Major Compounds from Glutamate-Induced Damage in PC12 Cells by MTT Assay
2.2.1. Cell Toxicity Induced by Glutamate
2.2.2. Effect of Single Compound on PC12 Cells under Different Concentrations
2.2.3. Effect of Six Compounds on Glutamate-Induced Damage in PC12 Cells
2.3. Effects of Myricitrin and Myricanol 11-Sulfate on Glutamate-Induced Apoptosis in PC12 Cells by Flow Cytometric Detection
2.4. Effect of Myricitrin and Myricanol 11-Sulfate on ROS, MDA and SOD Levels on Glutamate-Induced Oxidative Stress in PC12 Cells
2.5. MRB Is a Potential Natural Source of Neuroprotection
3. Materials and Methods
3.1. Drugs and Chemicals
3.2. Materials
3.3. Sample Preparations
3.4. UPLC-DAD and UPLC-ESI-Q-TOF-MS Analysis
3.5. Cell Culture and Treatment
3.6. Measurement of Cell Viability
3.7. Annexin V/PI Double Staining
3.8. ROS, MDA and SOD Assays
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corona, J.C.; Tovar-y-Romo, L.B.; Tapia, R. Glutamate excitotoxicity and therapeutic targets for amyotrophic lateral sclerosis. Expert Opin. Ther. Targets 2007, 11, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Fukui, M.; Zhu, B.T. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radical Biol. Med. 2010, 48, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, C.; Zhang, B.; Ferguson, I.B. Red bayberry: Botany and horticulture. Hortic. Rev. 2004, 30, 83–114. [Google Scholar]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Reference Publications Inc.: Algonac, MI, USA, 1985; Volume 2, p. 450. [Google Scholar]
- Nonaka, G.-I.; Muta, M.; Nishioka, I. Myricatin, a galloyl flavanonol sulfate and prodelphinidin gallates from Myrica rubra. Phytochemistry 1983, 22, 237–241. [Google Scholar] [CrossRef]
- Sakurai, N.; Yaguchi, Y.; Hirakawa, T.; Nagai, M.; Inoue, T. Two myricanol glycosides from Myrica rubra and revision of the structure of isomyricanone. Phytochemistry 1991, 30, 3077–3079. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yamakami, S.; Amakura, Y.; Yoshida, T. Diarylheptanoid Sulfates and Related Compounds from Myrica rubra Bark. J. Nat. Prod. 2012, 75, 1798–1802. [Google Scholar] [CrossRef]
- Sakurai, N.; Yaguchi, Y.; Inoue, T. Triterpenoids from Myrica rubra. Phytochemistry 1986, 26, 217–219. [Google Scholar] [CrossRef]
- Shen, S.-N.; Xia, F.-B.; Li, H.; Liu, Y.-M.; Pan, R.-L. A new diphenyl heptane compound in the bark of bayberry. J. Pharm. Sci. 2015, 6, 746–748. [Google Scholar]
- Ohta, S.; Sakurai, N.; Kamogawa, A.; Yaguchi, Y.; Inoue, T.; Shinoda, M. Protective effects of the bark of Myrica rubra Sieb. et Zucc. on experimental liver injuries. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1992, 112, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Yamazaki, M.; Matsuo, K.; Asanuma, Y.; Kubo, M. Anti-androgenic Activity of Myricae Cortex. Isolation of Active Constituents from Bark of Myrica rubra. Biol. Pharm. Bull. 2001, 24, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Fujita, Y.; Banno, N.; Watanabe, K.; Kimura, Y.; Manosroi, A.; Manosroi, J.; Akihisa, T. Three New Cyclic Diarylheptanoids and Other Phenolic Compounds from the Bark of Myrica rubra and Their Melanogenesis Inhibitory and Radical Scavenging Activities. J. Oleo Sci. 2010, 59, 213–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.; Morikawa, T.; Toguchida, I.; Ando, S.; Matsuda, H.; Yoshikawa, M. Inhibitors of nitric oxide production from the bark of Myrica rubra: structures of new biphenyl type diarylheptanoid glycosides and taraxerane type triterpene. Bioorg. Med. Chem. 2002, 10, 4005–4012. [Google Scholar] [CrossRef]
- Meotti, F.C.; Senthilmohan, R.; Harwood, D.T.; Missau, F.C.; Pizzolatti, M.G.; Kettle, A.J. Myricitrin as a substrate and inhibitor of myeloperoxidase: Implications for the pharmacological effects of flavonoids. Free Radical Biol. Med. 2008, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, A.; Moriwaki, M. Myricitrin degraded by simulated digestion inhibits oxidation of human low-density lipoprotein. Biosci. Biotechnol. Biochem. 2005, 69, 693–699. [Google Scholar] [CrossRef]
- Meotti, F.C.; Missau, F.C.; Ferreira, J.; Pizzolatti, M.G.; Mizuzaki, C.; Nogueira, C.W.; Santos, A.R.S. Anti-allodynic property of flavonoid myricitrin in models of persistent inflammatory and neuropathic pain in mice. Biochem. Pharmacol. 2006, 72, 1707–1713. [Google Scholar] [CrossRef]
- Meotti, F.C.; Posser, T.; Missau, F.C.; Pizzolatti, M.G.; Leal, R.B.; Santos, A.R.S. Involvement of p38 MAPK on the antinociceptive action of myricitrin in mice. Biochem. Pharmacol. 2007, 74, 924–931. [Google Scholar] [CrossRef]
- Meotti, F.C.; Fachinetto, R.; Maffi, L.C.; Missau, F.C.; Pizzolatti, M.G.; Rocha, J.B.T.; Santos, A.R.S. Antinociceptive action of myricitrin: involvement of the K+ and Ca2+ channels. Eur. J. Pharmacol. 2007, 567, 198–205. [Google Scholar] [CrossRef]
- Qin, M.; Luo, Y.; Meng, X.-B.; Wang, M.; Wang, H.-W.; Song, S.-Y.; Ye, J.-X.; Pan, R.-L.; Yao, F.; Wu, P.; et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascular Pharmacol. 2015, 70, 23–34. [Google Scholar] [CrossRef]
- Sun, G.-B.; Qin, M.; Ye, J.-X.; Pan, R.-L.; Meng, X.-B.; Wang, M.; Luo, Y.; Li, Z.-Y.; Wang, H.-W.; Sun, X.-B. Inhibitory effects of myricitrin on oxidative stress-induced endothelial damage and early atherosclerosis in ApoE−/− mice. Toxicol. Appl. Pharmacol. 2013, 271, 114–126. [Google Scholar] [CrossRef] [PubMed]
- JECFA2014a, Compendium of food additive Specifications. In: Joint FAO/WHO Expert Committee on Food Additives, 79th Meeting 2014. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwj1gM7j34vkAhUPwosBHXF8B5IQFjAAegQIARAC&url=http%3A%2F%2Fwww.fao.org%2F3%2Fa-i4144e.pdf&usg=AOvVaw3nlcJMnqIG9bSNW9ZvJs_v (accessed on 18 August 2019).
- JECFA2014b, Evaluation of certain food additives. In: Seventy-ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series; no. 990. Available online: https://www.google.com/search?newwindow=1&ei=tupYXcCAPNn4hwOOr7noAw&q=JECFA2014b%2C+Evaluation+of+certain+food+additives.+WHO+Technical+Report+Series%3B+no.+990&oq=JECFA2014b%2C+Evaluation+of+certain+food+additives.+WHO+Technical+Report+Series%3B+no.+990&gs_l=psy-ab.12...15477.16476..17204...0.0..0.215.369.0j1j1......0....1j2..gws-wiz.......33i299j33i160.mD2vHYONxqU&ved=0ahUKEwjAs9qb4IvkAhVZ_GEKHY5XDj0Q4dUDCAo (accessed on 18 August 2019).
- Aguilar, M.I.; Rovelo, R.; Verjan, J.G.; Lllescas, O.; Baeza, A.E.; Fuente, M.D.L.; Avila, L.; Navarrete, A. Anti-inflammatory activities, triterpenoids, and diarylheptanoids of ssp. Arguta. Pharm. Biol. 2011, 49. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.-F.; Xu, Y.; Lam, R.Y.Y.; Schneider, B.; Dou, H.; Leung, P.S.; Shi, S.-Y.; Zhou, C.-X.; Yang, L.-X.; Zhang, R.-P.; et al. Diarylheptanoids and a Monoterpenoid from the Rhizomes of Zingiber officinale: Antioxidant and Cytoprotective Properties. J. Nat. Prod. 2008, 71, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Kozuka, M.; Wang, H.K.; Konoshima, T.; Tokuda, H.; Okuda, M.; Mou, X.-Y.; Nishino, H.; Sakurai, N.; Lee, K.H.; et al. Antitumor-promoting effects of cyclic diarylheptanoids on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2000, 159, 135–140. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, M.R.; Shah, N.A.; Shah, S.A.; Ismail, H.; Younis, T.; Zahra, Z. Phytochemical, antioxidant and hepatoprotective effects of Alnus nitida bark, in carbon tetrachloride challenged Sprague Dawley rats. BMC Complementary Altern. Med. 2016, 16, 268. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discovery Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Grande, P.D.; Ninfali, P. Enhancement of flavonoid ability to cross the blood–brain barrier of rats by co-administration with α-tocopherol. Food Funct. 2014, 6, 394–400. [Google Scholar] [CrossRef]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of Dual Drug Loaded Lipid Based Nanoparticlesacross the Blood–Brain Barrier Impart Enhanced Neuroprotectionin a Rotenone Induced Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2016, 7, 1658–1670. [Google Scholar] [CrossRef]
- Dai, G.-H.; Meng, G.-M.; Tong, Y.-L.; Chen, X.; Ren, Z.-M.; Wang, K.; Yang, F. Growth-inhibiting and apoptosis-inducing activities of Myricanol from the bark of Myrica rubra in human lung adenocarcinoma A549 cells. Phytomedicine 2014, 21, 1490–1496. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Shen, S.-N.; Xia, F.-B.; Chang, Q.; Liu, X.-M.; Pan, R.-L. Neuroprotection of Stilbenes from Leaves of Cajanus cajan against Oxidative Damage Induced by Corticosterone and Glutamate in Differentiated PC12 Cells. Chin. Herbal Med. 2015, 7, 238–246. [Google Scholar] [CrossRef]
Sample Availability: Not available. |











| Peak No. | tR min | Molecular Formula | [M + H]+ m/z | MS/MS m/z | UV λnm | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 1.5 | C7H6O5 | 171.1212 | 127.0708 | 219.3, 271.1 | Gallic acid (std *) |
| 2 | 2.9 | C27H30O16 | 611.1321 | 303.0507, 153.0188 | 254.3, 353.8 | Rutin (std *) |
| 3 | 4.7 | C21H20O13 | 481.3438 | 319.0451, 153.0187 | 260.2, 316.2 | Myricetin hexoside |
| 4 | 5.0 | C21H20O12 | 465.0664 | 303.0526, 153.0208 | 261.7, 351.1 | Quercetin hexoside |
| 5 | 5.4 | C21H20O12 | 465.1031 | 319.0451, 153.0187 | 260.4, 359.8 | Myricitrin (std *) |
| 6 | 6.4 | C21H20O11 | 449.1089 | 303.0507, 229.0482, 153.0188 | 265.7, 338.1 | Quercetin deoxyhexoside |
| 7 | 6.8 | C15H10O8 | 319.0455 | 153.0187 | 252.9, 372.7 | Myricetin (std *) |
| 8 | 7.0 | C15H10O7 | 303.0502 | 153.0187 | 265.1, 365.9 | Quercetin (std *) |
| 9 | 7.2 | C15H10O6 | 287.0938 | 153.0187 | 265.7, 338.1 | Kaempferol |
| 10 | 8.0 | C27H36O10 | 521.2381 | 359.1859, 341.1768 | 216.2, 259.8, 305.1 | Myricanol hexoside |
| 11 | 8.5 | C27H36O10 | 521.2381 | 359.1859, 341.1768 | 204.1, 259.6, 293.4 | Myricanol hexoside |
| 12 | 8.8 | C27H36O10 | 521.2381 | 359.1859, 341.1768 | 222.4, 251.7, 294.7 | Myricanol hexoside |
| 13 | 9.7 | C21H26O8S | 439.1414 | 341.1750 | 213.2, 257.8, 295.3 | Myricanol 11-sulfate (std *) |
| 14 | 12.5 | C21H26O5 | 359.1870 | 341.1761 | 231.5, 259.0, 296.5 | Myricanol (std *) |
| 15 | 14.2 | C21H24O5 | 357.1840 | 339.1603, 325.1422 | 226.0, 258.4, 296.5 | Myricanone (std *) |
| 16 | 24.7 | C30H46O3 | 455.3525 | Uosolic Acid (std *) | ||
| 17 | 25.5 | C30H50O2 | 443.3889 | Myricadoil (std *) | ||
| 18 | 26.1 | C30H50O2 | 443.3889 | Uvaol (std *) | ||
| 19 | 26.4 | C30H50O | 427.3943 | Tarxerol (std *) |
| Group | Dose (μM) | ROS (% of Control) | MDA (nmol/mg Prot) | SOD (units/mg Prot) |
|---|---|---|---|---|
| Control | – | 100 ± 1 | 0.76 ± 0.1 | 32 ± 4 |
| Glutamate | 15 mM | 122 ± 6 ## | 2.64 ± 0.3 ## | 18 ± 1 ## |
| Myricanol 11-Sulfate | 5 | 110 ± 3 | 0.8 ± 0.1 ** | 30 ± 1 ** |
| Myricitrin | 10 | 100 ± 6 ** | 0.78 ± 0.1 ** | 38 ± 2 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Zhao, M.; Li, C.; Chang, Q.; Liu, X.; Liao, Y.; Pan, R. Study on the Material Basis of Neuroprotection of Myrica rubra Bark. Molecules 2019, 24, 2993. https://doi.org/10.3390/molecules24162993
Shen S, Zhao M, Li C, Chang Q, Liu X, Liao Y, Pan R. Study on the Material Basis of Neuroprotection of Myrica rubra Bark. Molecules. 2019; 24(16):2993. https://doi.org/10.3390/molecules24162993
Chicago/Turabian StyleShen, Shengnan, Mengjun Zhao, Chenchen Li, Qi Chang, Xinmin Liu, Yonghong Liao, and Ruile Pan. 2019. "Study on the Material Basis of Neuroprotection of Myrica rubra Bark" Molecules 24, no. 16: 2993. https://doi.org/10.3390/molecules24162993
APA StyleShen, S., Zhao, M., Li, C., Chang, Q., Liu, X., Liao, Y., & Pan, R. (2019). Study on the Material Basis of Neuroprotection of Myrica rubra Bark. Molecules, 24(16), 2993. https://doi.org/10.3390/molecules24162993