Pd Nanocatalyst Adorned on Magnetic Chitosan@N-Heterocyclic Carbene: Eco-Compatible Suzuki Cross-Coupling Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Synthesis of Functionalized-MWCNTs (f-MWCNTs)
2.4. Synthesis of Magnetic f-MWCNTs (m-f-MWCNTs)
2.5. Synthesis of the Cross-Linked Chitosan Using Imidazole
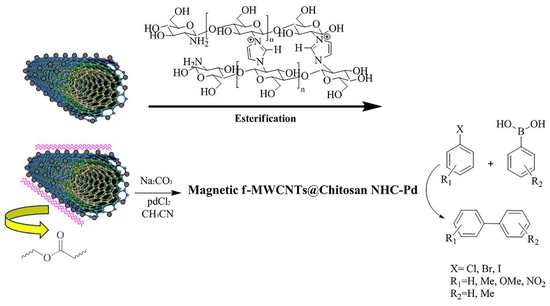
2.6. Substitution Cross-Linked Chitosan with Activated m-f-MWCNTs (Esterification)
2.7. Preparation of the m-f-MWCNTs@chitosan NHC-Pd
2.8. General Procedure for the Suzuki Reactions
3. Result and Discussion
3.1. Characterization of Nanocatalyst
3.1.1. FT-IR Spectroscopy
3.1.2. XPS Analysis
3.1.3. TGA Analysis
3.1.4. SEM and TEM Images and EDS Analysis
3.1.5. BET Analysis
3.2. Catalytic Performance of m-f-MWCNTs@chitosan NHC-Pd Nanocomposite
3.3. Study of Pd Leaching
3.4. The Reusability and Stability of Nanocatalyst
3.5. Comparison
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Magano, J.; Dunetz, J.R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Corbet, J.-P.; Mignani, G.; Corbet, J. Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev. 2006, 37, 2651–2710. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S.; Hiedari, B. Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki-Miyaura Cross-coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Barder, T.E.; Walker, S.D.; Martinelli, J.R.; Buchwald, S.L. Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005, 127, 4685–4696. [Google Scholar] [CrossRef] [PubMed]
- Miura, M. Rational Ligand Design in Constructing Efficient Catalyst Systems for Suzuki—Miyaura Coupling. Angew. Chem. Int. Ed. 2004, 35, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Marziale, A.N.; Jantke, D.; Faul, S.H.; Reiner, T.; Herdtweck, E.; Eppinger, J. An efficient protocol for the palladium-catalysed Suzuki–Miyaura cross-coupling. Green Chem. 2011, 13, 169–177. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jun, B.-H.; Byun, J.-W.; Lee, Y.-S. N-Heterocyclic carbene–palladium complex on polystyrene resin surface as polymer-supported catalyst and its application in Suzuki cross-coupling reaction. Tetrahedron Lett. 2004, 45, 5827–5831. [Google Scholar] [CrossRef]
- Li, J.-H.; Liu, W.-J. Dabco as an Inexpensive and Highly Efficient Ligand for Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reaction. Org. Lett. 2004, 6, 2809–2811. [Google Scholar] [CrossRef]
- Someya, H.; Ohmiya, H.; Yorimitsu, H.; Oshima, K. N-Heterocyclic Carbene Ligands in Cobalt-Catalyzed Sequential Cyclization/Cross-Coupling Reactions of 6-Halo-1-hexene Derivatives with Grignard Reagents. Org. Lett. 2007, 38, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Peris, E.; Crabtree, R.H. Recent homogeneous catalytic applications of chelate and pincer N-heterocyclic carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Group 11 Metal Complexes of N-Heterocyclic Carbene Ligands: Nature of the MetalCarbene Bond. Organometallics 2004, 23, 755–764. [Google Scholar] [CrossRef]
- Dorta, R.; Stevens, E.D.; Scott, N.M.; Costabile, C.; Cavallo, L.; Hoff, C.D.; Nolan, S.P. Steric and Electronic Properties of N-Heterocyclic Carbenes (NHC): A Detailed Study on Their Interaction with Ni(CO)4. J. Am. Chem. Soc. 2005, 127, 2485–2495. [Google Scholar] [CrossRef] [PubMed]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Seechurn, C.C.C.J.; Colacot, T.J. Development of Preformed Pd Catalysts for Cross-Coupling Reactions, Beyond the 2010 Nobel Prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Sydnes, M.O. The Use of Palladium on Magnetic Support as Catalyst for Suzuki–Miyaura Cross-Coupling Reactions. Catalysts 2017, 7, 35. [Google Scholar] [CrossRef]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A. Fe3O4 and MnO2 assembled on halloysite nanotubes: A highly efficient solid-phase extractant for electrochemical detection of mercury(II) ions. Sens. Actuators B Chem. 2016, 228, 1–9. [Google Scholar] [CrossRef]
- Guo, B.; Deng, F.; Zhao, Y.; Luo, X.; Luo, S.; Au, C.T. Magnetic ion-imprinted and –SH functionalized polymer for selective removal of Pb(II) from aqueous samples. Appl. Surf. Sci. 2014, 292, 438–446. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Luo, X.; Yang, L.; Tu, X. Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 395, 183–189. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Gao, J.; Dai, J.; Han, J.; Wang, Y.; Xie, J.; Yan, Y. Selective adsorption behavior of Pb(II) by mesoporous silica SBA-15-supported Pb(II)-imprinted polymer based on surface molecularly imprinting technique. J. Hazard. Mater. 2011, 186, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, N.M.; Senkal, B.F.; Cengiz, E.; Yaman, M. Novel Polymeric Resin for Solid Phase Extraction and Determination of Lead in Waters. CLEAN Soil Air Water 2010, 38, 1047–1054. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wang, Y.; Dai, J.; Gao, J.; Xie, J.; Yan, Y. A surface ion-imprinted mesoporous sorbent for separation and determination of Pb (II) ion by flame atomic absorption spectrometry. Microchim. Acta 2011, 172, 309–317. [Google Scholar] [CrossRef]
- Murugan, E.; Gopi, V. Amphiphilic Multiwalled Carbon Nanotube Polymer Hybrid with Improved Conductivity and Dispersibility Produced by Functionalization with Poly(vinylbenzyl)triethylammonium Chloride. J. Phys. Chem. C 2011, 115, 19897–19909. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Magnetically retrievable catalysts for organic synthesis. Chem. Commun. 2013, 49, 752–770. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.N.; Varma, R.S. Organic synthesis via magnetic attraction: Benign and sustainable protocols using magnetic nanoferrites. Green Chem. 2013, 15, 398–417. [Google Scholar] [CrossRef]
- Huang, K.; Xue, L.; Hu, Y.-C.; Huang, M.-Y.; Jiang, Y.-Y. Catalytic behaviors of silica-supported starch–polysulfosiloxane–Pt complexes in asymmetric hydrogenation of 4-methyl-2-pentanone. React. Funct. Polym. 2002, 50, 199–203. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, C.-G. Natural biopolymer-supported bimetallic catalyst system for the carbonylation to esters of Naproxen. J. Mol. Catal. A Chem. 2003, 206, 59–65. [Google Scholar] [CrossRef]
- Leonhardt, S.E.S.; Ondruschka, B.; Ondruschka, J. Comment on Aspects of Chitosan Preparation. Chem. Eng. Technol. 2008, 31, 917–921. [Google Scholar] [CrossRef]
- Agboh, O.; Qin, Y. Chitin and chitosan fibers. Polym. Adv. Technol. 1997, 8, 355–365. [Google Scholar] [CrossRef]
- Knaul, J.Z.; Hudson, S.M.; Creber, K.A.M. Improved mechanical properties of chitosan fibers. J. Appl. Polym. Sci. 1999, 72, 1721–1732. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres-Mendieta, R.; Novotný, V.; Nguyen, N.H.A.; Ševců, A.; Padil, V.V.T.; Müllerová, J.; Stuchlik, M.; et al. A poly(3-hydroxybutyrate)–chitosan polymer conjugate for the synthesis of safer gold nanoparticles and their applications. Green Chem. 2018, 20, 4975–4982. [Google Scholar] [CrossRef]
- Baba, Y.; Hirakawa, H.; Yoshizuka, K.; Inuoe, K.; Kawano, Y. Adsorption Equilibria of Silver(I) and Copper(II) Ions on N-(2-Hydroxylbenzyl)chitosan Derivative. Anal. Sci. 1994, 10, 601–605. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Heeres, J.; Backx, L.J.J.; Mostmans, J.H.; Van Cutsem, J. Antimycotic imidazoles. Part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J. Med. Chem. 1979, 22, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Debus, H. Ueber die einwirkung des ammoniaks auf glyoxal. Eur. J. Org. Chem. 1858, 107, 199–208. [Google Scholar] [CrossRef]
- Huong, L.T.T.; Nam, N.H.; Doan, D.H.; Nhung, H.T.M.; Quang, B.T.; Nam, P.H.; Thong, P.Q.; Phuc, N.X.; Thu, H.P. Folate attached, curcumin loaded Fe3O4 nanoparticles: A novel multifunctional drug delivery system for cancer treatment. Mater. Chem. Phys. 2016, 172, 98–104. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef] [Green Version]
- Sonogashira, K. Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Heck, R.F. Palladium-Catalyzed Vinylation of Organic Halides. Org. React. 1982, 345–390. [Google Scholar]
- Negishi, E.-I.; Hu, Q.; Huang, Z.; Qian, M.; Wang, G.; Brown, H. Palladium-catalyzed alkenylation by the Negishi coupling. Aldrichim. Acta 2005, 38, 71–87. [Google Scholar] [CrossRef]
- Chang, X.; Jiang, N.; Zheng, H.; He, Q.; Hu, Z.; Zhai, Y.; Cui, Y. Solid-phase extraction of iron(III) with an ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Talanta 2007, 71, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Rofouei, M.K.; Payehghadr, M.; Shamsipur, M.; Ahmadalinezhad, A. Solid phase extraction of ultra traces silver(I) using octadecyl silica membrane disks modified by 1,3-bis(2-cyanobenzene) triazene (CBT) ligand prior to determination by flame atomic absorption. J. Hazard. Mater. 2009, 168, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Sarker, R.B.; Bhuiyan, A. Electrical conduction mechanism in plasma polymerized 1-Benzyl-2-methylimidazole thin films under static electric field. Thin Solid Films 2011, 519, 5912–5916. [Google Scholar] [CrossRef]
- Silva, S.M.; Braga, C.R.; Fook, M.V.; Raposo, C.M.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech Open: Rijeka, Croatia, 2012. [Google Scholar] [Green Version]
- Chang, X.; Chen, D.; Jiao, X. Chitosan-Based Aerogels with High Adsorption Performance. J. Phys. Chem. B 2008, 112, 7721–7725. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Cui, Y. Catalytic performance of cellulose supported palladium complex for Heck reaction in water. J. Appl. Polym. Sci. 2008, 110, 2996–3000. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Zhang, W.; Yuan, G.; Xiong, R.; Zhang, X. A novel reagentless approach for synthesizing cellulose nanocrystal-supported palladium nanoparticles with enhanced catalytic performance. J. Mater. Chem. A 2013, 1, 8645–8652. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Wang, Y.; Zhou, Z.; Zhang, X. Nanofibrillated cellulose as the support and reductant for the facile synthesis of Fe3O4/Ag nanocomposites with catalytic and antibacterial activity. J. Mater. Chem. A 2013, 1, 14910–14918. [Google Scholar] [CrossRef]
- Paul, S.; Islam, M.M. Suzuki–Miyaura reaction by heterogeneously supported Pd in water: Recent studies. RSC Adv. 2015, 5, 42193–42221. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon—Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 38, 133–173. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.-I. Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-like Macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Hong, M.C.; Choi, M.C.; Chang, Y.W.; Lee, Y.; Kim, J.; Rhee, H. ChemInform Abstract: Palladium Nanoparticles on Thermoresponsive Hydrogels and Their Application as Recyclable Suzuki-Miyaura Coupling Reaction Catalysts in Water. Adv. Synth. Catal. 2012, 43, 1257–1263. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous Catalysts for Liquid-Phase Oxidations: Philosophers’ Stones or Trojan Horses? Accounts Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Leazer, J.; Varma, R.S. Magnetically separable Fe3O4@DOPA–Pd: A heterogeneous catalyst for aqueous Heck reaction. Clean Technol. Environ. Policy 2015, 17, 2073–2077. [Google Scholar] [CrossRef]
- Sá, S.; Gawande, M.B.; Velhinho, A.; Veiga, J.P.; Bundaleski, N.; Trigueiro, J.; Tolstoguzov, A.; Teodoro, O.M.N.D.; Zbořil, R.; Varma, R.S.; et al. Magnetically recyclable magnetite–palladium (Nanocat-Fe–Pd) nanocatalyst for the Buchwald–Hartwig reaction. Green Chem. 2014, 16, 3494–3500. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Lee, Y.; Hong, M.C.; Ahn, H.; Yu, J.; Rhee, H. Pd nanoparticles immobilized on poly(NIPAM-co-4-VP) hydrogel: Highly active and reusable catalyst for carbon–carbon coupling reactions in water. J. Organomet. Chem. 2014, 769, 80–93. [Google Scholar] [CrossRef]
- Shang, N.; Feng, C.; Zhang, H.; Gao, S.; Tang, R.; Wang, C.; Wang, Z. Suzuki–Miyaura reaction catalyzed by graphene oxide supported palladium nanoparticles. Catal. Commun. 2013, 40, 111–115. [Google Scholar] [CrossRef]
- Shang, N.; Gao, S.; Feng, C.; Zhang, H.; Wang, C.; Wang, Z. Graphene oxide supported N-heterocyclic carbene-palladium as a novel catalyst for the Suzuki–Miyaura reaction. RSC Adv. 2013, 3, 21863–21868. [Google Scholar] [CrossRef]
- Song, H.-Q.; Zhu, Q.; Zheng, X.-J.; Chen, X.-G. One-step synthesis of three-dimensional graphene/multiwalled carbon nanotubes/Pd composite hydrogels: An efficient recyclable catalyst for Suzuki coupling reactions. J. Mater. Chem. A 2015, 3, 10368–10377. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhang, X.; Bian, F.; Yu, W. A novel thermo and pH-double sensitive hydrogel immobilized Pd catalyst for Heck and Suzuki reactions in aqueous media. React. Funct. Polym. 2012, 72, 233–241. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, J.; Wang, J.; Li, W.; Kaliaguine, S.; Hou, X.; Deng, Y.; Zhao, D. A versatile designed synthesis of magnetically separable nano-catalysts with well-defined core–shell nanostructures. J. Mater. Chem. A 2014, 2, 6071–6074. [Google Scholar] [CrossRef]
- Woo, H.; Lee, K.; Park, J.C.; Park, K.H. Facile synthesis of Pd/Fe3O4 /charcoal bifunctional catalysts with high metal loading for high product yields in Suzuki–Miyaura coupling reactions. New J. Chem. 2014, 38, 5626–5632. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







| Entry | Amount of Catalyst (mol%) | T (°C) | Solvent | Time (h) | Conversion (%) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 80 | H2O | 5 | 80 | 75 |
| 2 | 0.5 | 80 | H2O | 5 | 90 | 84 |
| 3 | 0.5 | 80 | H2O-EtOH | 5 | 100 | 97 |
| 4 | 0.3 | 80 | H2O-EtOH | 5 | 98 | 94 |
| 5 | 0.1 | 80 | H2O-EtOH | 5 | 97 | 94 |
| 6 | 0.1 | 50 | H2O-EtOH | 3 | 97 | 94 |
| 7 | 0.08 | 50 | H2O-EtOH | 3 | 85 | 80 |

| Entry | R1 | R2 | X | Amount of Catalyst (mol%) | Time (h) | Temperature (°C) | Conversion (%) | Yield (%) | TOF a | TON |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | H | I | 0.1 | 2 | 40 | 100 | 97 | 485 | 970 |
| 2 | 4-Me | H | I | 0.1 | 2 | 40 | 100 | 97 | 485 | 970 |
| 3 | 4-OMe | H | I | 0.1 | 2 | 40 | 100 | 97 | 485 | 970 |
| 4 | H | H | Br | 0.1 | 3 | 50 | 97 | 94 | 313 | 939 |
| 5 | 4-NO2 | H | Br | 0.1 | 3 | 50 | 98 | 95 | 316 | 948 |
| 6 | 4-Me | H | Br | 0.1 | 3 | 50 | 94 | 91 | 303 | 909 |
| 7 | H | H | Cl | 1 | 5 | 50 | N.R. | - | - | - |
| 8 | H | H | Cl | 1 | 8 | 60 | 60 | 53 | 6.6 | 52.8 |
| 9 | 4-Me | H | Cl | 1 | 8 | 60 | 53 | 50 | 6.25 | 50 |
| 10 | 4-NO2 | H | Cl | 1 | 8 | 60 | 68 | 62 | 7.75 | 62 |
| 11 | H | Me | I | 0.1 | 2 | 40 | 98 | 95 | 475 | 950 |
| 12 | 4-Me | Me | I | 0.1 | 2 | 40 | 97 | 94 | 470 | 940 |
| 13 | 4-OMe | Me | I | 0.1 | 2 | 40 | 96 | 93 | 465 | 930 |
| 14 | H | Me | Br | 0.1 | 3 | 50 | 92 | 89 | 296 | 888 |
| 15 | 4-Me | Me | Br | 0.1 | 3 | 50 | 89 | 83 | 276 | 828 |
| 16 | H | Me | Cl | 1 | 8 | 60 | 55 | 50 | 6.25 | 50 |
| 17 | 4-Me | Me | Cl | 1 | 8 | 60 | 50 | 47 | 5.87 | 46.96 |
| 18 | 4-OMe | Me | Cl | 1 | 8 | 60 | 45 | 40 | 5 | 40 |
| Entry | Catalyst (Pd Loading (mol%)) | Reaction Conditions | Time (h) | Yield or Conversion (%) | Reusability | Ref. |
|---|---|---|---|---|---|---|
 | ||||||
| 1 | poly(NIPAM-co-4-VP)-Pd (1) | K2CO3, H2O, 60 °C | 5 | 95 | 5 times | [58] |
| 2 | GO-NH2-Pd(II) (1) | K2CO3, EtOH:H2O, 60 °C | 4 | 71 | 10 times | [59] |
| 3 | GO-NHC-Pd(II) (0.25) | K2CO3, EtOH:H2O, 80 °C | 20 | 94 | 6 times | [60] |
| 4 | rGO/Pd (0.5) | K2CO3, EtOH:H2O, 60 °C | 1.5 | 71 | 6 times | [61] |
| 5 | m-f-MWCNTs@chitosan NHC-Pd nanocomposite (0.1) | K2CO3, EtOH:H2O, 50 °C | 3 | 89 | 5 times. | This work |
 | ||||||
| 6 | Poly(NIPAM-4-VP AC)-Pd (0.05), X = Br | K2CO3, H2O, Ar atmosphere, 90 °C | 1 | 92 | 6 times | [62] |
| 7 | GO-NH2-Pd(II) (1), X = Br | K2CO3, EtOH:H2O, 60 °C | 4 | 73 | 10 times | [59] |
| 8 | Fe3O4@C-Pd@mSiO2 (1.5), X = I | K2CO3, iPrOH, 70 °C | 6 | 92 | 6 times | [63] |
| 9 | Pd@Fe3O4@C (1), X = Br | K2CO3, DMF:H2O, 100 °C | 4 | 99 | - | [64] |
| 10 | m-f-MWCNTs@chitosan NHC-Pd (0.1), X = Br | K2CO3, EtOH:H2O, 50 °C | 3 | 94 | 5 times | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedghi, R.; Heidari, B.; Shahmohamadi, H.; Zarshenas, P.; Varma, R.S. Pd Nanocatalyst Adorned on Magnetic Chitosan@N-Heterocyclic Carbene: Eco-Compatible Suzuki Cross-Coupling Reaction. Molecules 2019, 24, 3048. https://doi.org/10.3390/molecules24173048
Sedghi R, Heidari B, Shahmohamadi H, Zarshenas P, Varma RS. Pd Nanocatalyst Adorned on Magnetic Chitosan@N-Heterocyclic Carbene: Eco-Compatible Suzuki Cross-Coupling Reaction. Molecules. 2019; 24(17):3048. https://doi.org/10.3390/molecules24173048
Chicago/Turabian StyleSedghi, Roya, Bahareh Heidari, Hatef Shahmohamadi, Pourya Zarshenas, and Rajender S. Varma. 2019. "Pd Nanocatalyst Adorned on Magnetic Chitosan@N-Heterocyclic Carbene: Eco-Compatible Suzuki Cross-Coupling Reaction" Molecules 24, no. 17: 3048. https://doi.org/10.3390/molecules24173048
APA StyleSedghi, R., Heidari, B., Shahmohamadi, H., Zarshenas, P., & Varma, R. S. (2019). Pd Nanocatalyst Adorned on Magnetic Chitosan@N-Heterocyclic Carbene: Eco-Compatible Suzuki Cross-Coupling Reaction. Molecules, 24(17), 3048. https://doi.org/10.3390/molecules24173048