Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S
Abstract
:1. Introduction
2. Results and Discussion
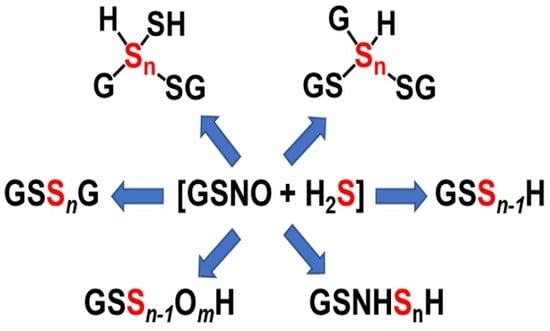
2.1. Reaction Methodology and Overview
2.2. Characterization of Glutathione Polysulfanes (GSSnSG)
2.3. Glutathione Polysulfides GSSnH and Alkylated Derivatives
2.4. Characterization of Glutathione Oxoacids, Sulfenic, Sulfinic, and Sulfonic
2.5. Mixed Valence Sulfide Products



2.6. N-Containing GSX
2.7. Competitive Trapping Experiments
2.8. Role of S Radicals in Polysulfane Formation

3. Materials and Methods
3.1. General Protocol for Orbitrap LC-HRMS Analysis
3.2. Reaction Protocols
3.3. Relative Distribution Calculations
3.4. Reactions of GSNO with H2S
3.5. Radical Trapping Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, R. The Gasotransmitter Role of Hydrogen Sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M.; Carrington, S.J.; Tantillo, D.J.; Harrison, J.G.; Ignarro, L.J.; Freeman, B.A.; Chen, A.; Wink, D.A. Small Molecule Signaling Agents: The Integrated Chemistry and Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chem. Res. Toxicol. 2012, 25, 769–793. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shen, X.; Kevil, C.G. A Tale of Two Gases: NO and H2S, Foes or Friends for Life? Redox Biol. 2013, 1, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Bruce King, S. Potential Biological Chemistry of Hydrogen Sulfide (H2S) with the Nitrogen Oxides. Free Radic. Biol. Med. 2013, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Akaike, T.; Sawa, T.; Kumagai, Y.; Wink, D.A.; Tantillo, D.J.; Hobbs, A.J.; Nagy, P.; Xian, M.; Lin, J.; et al. Redox Chemistry and Chemical Biology of H2S, Hydropersulfides, and Derived Species: Implications of Their Possible Biological Activity and Utility. Free Radic. Biol. Med. 2014, 77, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides Are Possible H2S-Derived Signaling Molecules in Rat Brain. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 2451–2457. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kuhnle, G.G.C.; Dyson, A.; Fernandez, B.O.; Grman, M.; DuMond, J.F.; Barrow, M.P.; McLeod, G.; Nakagawa, H.; Ondrias, K.; et al. Key Bioactive Reaction Products of the NO/H2S Interaction Are S/N-Hybrid Species, Polysulfides, and Nitroxyl. Proc. Natl. Acad. Sci. USA 2015, 112, E4651–4660. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; De La Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO Cooperatively Regulate Vascular Tone by Activating a Neuroendocrine HNO-TRPA1-CGRP Signalling Pathway. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Smith, B.C.; Marletta, M.A. Mechanisms of S-Nitrosothiol Formation and Selectivity in Nitric Oxide Signaling. Curr. Opin. Chem. Biol. 2012, 16, 498–506. [Google Scholar] [CrossRef]
- Grman, M.; Nasim, M.J.; Leontiev, R.; Misak, A.; Jakusova, V.; Ondrias, K.; Jacob, C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants 2017, 6, 14. [Google Scholar] [CrossRef]
- Kimura, H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxid. Redox Signal. 2015, 22, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Koike, S.; Shibuya, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Potential Redox Regulators Cysteine- and Glutathione-Persulfide (Cys-SSH and GSSH) Together with Signaling Molecules H2S2, H2S3 and H2S. Sci. Rep. 2017, 7, 10459. [Google Scholar] [CrossRef]
- Akaike, T.; Ida, T.; Wei, F.-Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-TRNA Synthetase Governs Cysteine Polysulfidation and Mitochondrial Bioenergetics. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Nishimura, A.; Nasuno, R.; Yoshikawa, Y.; Jung, M.; Ida, T.; Matsunaga, T.; Morita, M.; Takagi, H.; Motohashi, H.; Akaike, T. Mitochondrial Cysteinyl-TRNA Synthetase Is Expressed via Alternative Transcriptional Initiation Regulated by Energy Metabolism in Yeast Cells. J. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kunieda, K.; Kitamura, A.; Kakihana, Y.; Akaike, T.; Ihara, H. 8-Nitro-CGMP Attenuates the Interaction between SNARE Complex and Complexin through S-Guanylation of SNAP-25. ACS Chem. Neurosci. 2018, 9, 217–223. [Google Scholar] [CrossRef]
- Kunieda, K.; Tsutsuki, H.; Ida, T.; Kishimoto, Y.; Kasamatsu, S.; Sawa, T.; Goshima, N.; Itakura, M.; Takahashi, M.; Akaike, T.; et al. 8-Nitro-CGMP Enhances SNARE Complex Formation through S-Guanylation of Cys90 in SNAP25. ACS Chem. Neurosci. 2015, 6, 1715–1725. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen Sulfide, an Enhancer of Vascular Nitric Oxide Signaling: Mechanisms and Implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef]
- Atwood, D.A.; Zaman, M.K. Sulfur: Organic Polysulfanes Based in Part on the Article Sulfur: Organic Polysulfanes by Ralf Steudel & Monika Kustos Which Appeared in the Encyclopedia of Inorganic Chemistry, First Edition. Encyclopedia Inorg. Bioinorg. Chem. 2011. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of Reactive Sulfur Species for Signaling by Hydrogen Sulfide Oxidation Pathways. Nat. Chem. Biol. 2015, 11, 457–464. [Google Scholar] [CrossRef]
- Kumar, M.R.; Farmer, P.J. Chemical Trapping and Characterization of Small Oxoacids of Sulfur (SOS) Generated in Aqueous Oxidations of H2S. Redox Biol. 2017, 112, 62. [Google Scholar] [CrossRef]
- Makarov, S.V.; Horváth, A.K.; Makarova, A.S. Reactivity of Small Oxoacids of Sulfur. Mol. Basel Switz. 2019, 24. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-NITROSOGLUTATHIONE. Biochim. Biophys. Acta 2013, 1830, 3173–3181. [Google Scholar] [CrossRef]
- Corpas, F.J.; Alché, J.D.; Barroso, J.B. Current Overview of S-Nitrosoglutathione (GSNO) in Higher Plants. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Lee, U.; Wie, C.; Fernandez, B. O.; Feelisch, M.; Vierling, E. Modulation of Nitrosative Stress by S-Nitrosoglutathione Reductase Is Critical for Thermotolerance and Plant Growth in Arabidopsis. Plant Cell 2008, 20, 786–802. [Google Scholar] [CrossRef]
- Grman, M.; Misak, A.; Jacob, C.; Tomaskova, Z.; Bertova, A.; Burkholz, T.; Docolomansky, P.; Habala, L.; Ondrias, K. Low Molecular Thiols, PH and O2 Modulate H2S-Induced S-Nitrosoglutathione Decomposition–•NO Release. Gen. Physiol. Biophys. 2013, 32, 429–441. [Google Scholar] [CrossRef]
- Hogg, N.; Singh, R.J.; Kalyanaraman, B. The Role of Glutathione in the Transport and Catabolism of Nitric Oxide. FEBS Lett. 1996, 382, 223–228. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Santos, J.L.T.; Mergia, E.; Grman, M.; Nagy, P.; Kelm, M.; Butler, A.; Feelisch, M. Nitrosopersulfide (SSNO−) Accounts for Sustained NO Bioactivity of S-Nitrosothiols Following Reaction with Sulfide. Redox Biol. 2014, 2, 234–244. [Google Scholar] [CrossRef]
- Wedmann, R.; Ivanovic-Burmazovic, I.; Filipovic, M.R. Nitrosopersulfide (SSNO−) Decomposes in the Presence of Sulfide, Cyanide or Glutathione to Give HSNO/SNO−: Consequences for the Assumed Role in Cell Signalling. Interface Focus 2017, 7. [Google Scholar] [CrossRef]
- Wedmann, R.; Zahl, A.; Shubina, T.E.; Duerr, M.; Heinemann, F.W.; Bugenhagen, B.E.C.; Burger, P.; Ivanovic-Burmazovic, I.; Filipovic, M.R. Does Perthionitrite (SSNO-) Account for Sustained Bioactivity of NO? A (Bio)Chemical Characterization. Inorg. Chem. 2015, 54, 9367–9380. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the Chemical Biology of the Nitrite/Sulfide Interaction. Nitric Oxide-Biol. Chem. 2015, 46, 14–24. [Google Scholar] [CrossRef]
- Kumar, M.R.; Clover, T.; Olaitan, A.D.; Becker, C.; Solouki, T.; Farmer, P.J. The Reaction between GSNO and H2S: On the Generation of NO, HNO and N2O. Nitric Oxide 2018, 77, 96–105. [Google Scholar] [CrossRef]
- Seo, Y.H.; Carroll, K.S. Quantification of Protein Sulfenic Acid Modifications Using Isotope-Coded Dimedone and Iododimedone. Angew. Chem. Int. Ed Engl. 2011, 50, 1342–1345. [Google Scholar] [CrossRef]
- Charles, R.L.; Schröder, E.; May, G.; Free, P.; Gaffney, P.R.J.; Wait, R.; Begum, S.; Heads, R.J.; Eaton, P. Protein Sulfenation as a Redox Sensor: Proteomics Studies Using a Novel Biotinylated Dimedone Analogue. Mol. Cell. Proteomics 2007, 6, 1473–1484. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Leonard, S.E.; Reddie, K.G.; Carroll, K.S. Mining the Thiol Proteome for Sulfenic Acid Modifications Reveals New Targets for Oxidation in Cells. ACS Chem. Biol. 2009, 4, 783–799. [Google Scholar] [CrossRef]
- Singh, S.P.; Wishnok, J.S.; Keshive, M.; Deen, W.M.; Tannenbaum, S.R. The Chemistry of the S-Nitrosoglutathione/Glutathione System. Proc. Natl. Acad. Sci. USA 1996, 93, 14428–14433. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological Hydropersulfides and Related Polysulfides—A New Concept and Perspective in Redox Biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Kumar, M.R.; Farmer, P.J. Trapping Reactions of the Sulfenyl and Sulfinyl Tautomers of Sulfenic Acids. ACS Chem. Biol. 2016, 12, 474–478. [Google Scholar] [CrossRef]
- Freeman, F. Mechanisms of Reactions of Sulfur Hydride Hydroxide: Tautomerism, Condensations, and C-Sulfenylation and O-Sulfenylation of 2,4-Pentanedione. J. Phys. Chem. A 2015, 119, 3500–3517. [Google Scholar] [CrossRef]
- Steudel, R. The Chemistry of Organic Polysulfanes R-Sn-R (n > 2). Chem. Rev. 2002, 102, 3905–3946. [Google Scholar] [CrossRef]
- Shoeman, D.W.; Nagasawa, H.T. The Reaction of Nitroxyl (HNO) with Nitrosobenzene Gives Cupferron (N-Nitrosophenylhydroxylamine). Nitric Oxide Biol. Chem. 1998, 2, 66–72. [Google Scholar] [CrossRef]
- Johnson, G.M.; Chozinski, T.J.; Gallagher, E.S.; Aspinwall, C.A.; Miranda, K.M. Glutathione Sulfinamide Serves as a Selective, Endogenous Biomarker for Nitroxyl after Exposure to Therapeutic Levels of Donors. Free Radic. Biol. Med. 2014, 76, 299–307. [Google Scholar] [CrossRef]
- Hagen, M.; Schiffels, P.; Hammer, M.; Dörfler, S.; Tübke, J.; Hoffmann, M.J.; Althues, H.; Kaskel, S. In-Situ Raman Investigation of Polysulfide Formation in Li-S Cells. J. Electrochem. Soc. 2013, 160, A1205–A1214. [Google Scholar] [CrossRef]
- Bianco, C.L.; Chavez, T.A.; Sosa, V.; Simran, S.S.; Nguyen, Q.N.N.; Tantillo, D.J.; Ichimura, A.S.; Toscano, J.P.; Fukuto, J.M. The chemical biology of the persulfide (RSSH)/perthiyl (RSS·) redox couple and possible role in biological redox signaling. Free Radic. Biol. Med. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Singh, R.J.; Hogg, N.; Joseph, J.; Kalyanaraman, B. Mechanism of Nitric Oxide Release from S-Nitrosothiols. J. Biol. Chem. 1996, 271, 18596–18603. [Google Scholar] [CrossRef] [Green Version]
- Ortiz de Montellano, P.R.; Stearns, R.A. Timing of the Radical Recombination Step in Cytochrome P-450 Catalysis with Ring-Strained Probes. J. Am. Chem. Soc. 1987, 109, 3415–3420. [Google Scholar] [CrossRef]
- Bianco, C.L.; Akaike, T.; Ida, T.; Nagy, P.; Bogdandi, V.; Toscano, J.P.; Kumagai, Y.; Henderson, C.F.; Goddu, R.N.; Lin, J.; et al. The Reaction of Hydrogen Sulfide with Disulfides: Formation of a Stable Trisulfide and Implications for Biological Systems. Br. J. Pharmacol. 2019, 176, 671–683. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.R.; Farmer, P.J. Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S. Molecules 2019, 24, 3090. https://doi.org/10.3390/molecules24173090
Kumar MR, Farmer PJ. Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S. Molecules. 2019; 24(17):3090. https://doi.org/10.3390/molecules24173090
Chicago/Turabian StyleKumar, Murugaeson R, and Patrick J Farmer. 2019. "Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S" Molecules 24, no. 17: 3090. https://doi.org/10.3390/molecules24173090
APA StyleKumar, M. R., & Farmer, P. J. (2019). Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S. Molecules, 24(17), 3090. https://doi.org/10.3390/molecules24173090