Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria
Abstract
:1. Introduction
2. What is the Threat?
- Massive and global public awareness campaign;
- Improve hygiene and prevent the spread of infections;
- Reduce the (unnecessary) use of antimicrobials in agriculture and their dissemination in the environment;
- Improve global surveillance for (i) antimicrobial resistance and (ii) antimicrobial consumption in humans and animals;
- Promote new and rapid diagnostic tests to stop the use of antibiotics as quickly as possible;
- Promote the development and use of vaccines and (therapeutic) alternatives;
- Improve the number, remuneration, and recognition of people working in infectious diseases;
- Establish a global innovation fund for pre-clinical and non-commercial research;
- Promote better investment for new medicines and improve existing ones;
- Build a global coalition for real action - via the G20 and the UN.
3. How did We Get There?
4. Is the War Lost?
- ✓
- ceftolozane (3rd generation cephalosporin) + tazobactam (β-lactamase inhibitor): combination which has a rather broad antibacterial spectrum: Pseudomonas aeruginosa, Enterobacteriaceae responsible for community infections (Escherichia coli, Proteus mirabilis, Proteus vulgaris, Salmonella spp.) and Enterobacteriaceae producing cephalosporinases responsible for nosocomial infections (Citrobacter freundii, Morganella morganii, and Serratia marcescens). On the other hand, this combination is less active on extended-spectrum β-lactamase-producing (ESBL) Klebsiella pneumoniae strains and ceftazidime-resistant Enterobacter spp. strains (i.e., the strains which overexpressed AmpC) [26].
- ✓
- ceftazidime (3rd generation cephalosporin) + avibactam (non-β-lactam β-lactamase inhibitor): avibactam is a β-lactamase inhibitor of a new class (i.e., diazabicyclooctanones) which has a broader inhibitory activity than “classical” β-lactamase inhibitors. It inhibits both class A and class C enzymes (i.e., Ambler classification), including extended spectrum β-lactamases (ESBL), KPC and OXA-48 carbapenemases, and AmpC enzymes. However, it has no effect on class B enzymes (metallo-β-lactamases) and is not capable of inhibiting many class D enzymes [27].
- ✓
- meropenem (carbapenem) + vaborbactam (non-β-lactam β-lactamase inhibitor): vaborbactam, a β-lactamase inhibitor of a new class (cyclic boronates), prevents certain classes of β-lactamases (class A and class C) from hydrolyzing meropenem and therefore restores its activity in many infections due to carbapenem-resistant Enterobacteriaceae. [28].
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Infectious Diseases Society of America. Bag Bugs, No Drugs: As Antibiotic Discovery Stagnates, A Public Health Crisis Brews. Available online: https://www.idsociety.org/globalassets/idsa/policy--advocacy/current_topics_and_issues/antimicrobial_resistance/10x20/statements/070104-as-antibiotic-discovery-stagnates-a-public-health-crisis-brews.pdf (accessed on 15 August 2019).
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Infectious Diseases Society of America. The 10 × ’20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 2010, 50, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/fr/ (accessed on 15 August 2019).
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 15 August 2019).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 15 August 2019).
- Review on Antimicrobial Resistance. Available online: https://amr-review.org/ (accessed on 15 August 2019).
- Eurostat. Statistics Explained. 2016. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/File:Demographic_balance,_2016_%28thousands%29.png (accessed on 15 August 2019).
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K., Jr.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. Infectious Diseases Society of America. 10 × ’20 Progress—development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Drug Approvals and Databases. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/default.htm (accessed on 15 August 2019).
- Center Watch. FDA Approved Drugs for Infections and Infectious Diseases. Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/25/infections-and-infectious-diseases (accessed on 15 August 2019).
- Grare, M. De la Genèse d’une Nouvelle Classe d’Antibactériens à Base de Polyphénols Cycliques de Type Calixarène: Études Moléculaire(s), Cellulaires(s) et Structurale(s) en Vue de l’Identification des Cibles d’Action: Le Cas du Para-Guanidinoéthylcalix[4]arène. Ph.D. Thesis, Université de Lorraine, Nancy, France, 3 June 2009. Available online: http://docnum.univ-lorraine.fr/public/SCD_T_2009_0138_GRARE.pdf (accessed on 15 August 2019).
- Trémolières, F.; Garraffo, R.; Lortholary, O. Nécessité d’approches alternatives dans les stratégies d’évaluation des nouveaux antibiotiques antibactériens. Med. Mal. Infect. 2005, 35 (Suppl. S3), S229–S235. [Google Scholar] [CrossRef]
- Johnson, A.P.; Uttley, A.H.; Woodford, N.; George, R.C. Resistance to vancomycin and teicoplanin: An emerging clinical problem. Clin. Microbiol. Rev. 1990, 3, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Raoult, D. Colistin: An antimicrobial for the 21st century? Clin. Infect. Dis. 2002, 35, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, H.; Le Monnier, A.; Carbonnelle, E.; Corvec, S.; Illiaquer, M.; Bille, E.; Zahar, J.R.; Jauréguy, F.; Fihman, V.; Tankovic, J.; et al. Gmc Study Group. In vitro antimicrobial activity of “last-resort” antibiotics against unusual nonfermenting Gram-negative bacilli clinical isolates. Microb. Drug Resist. 2012, 18, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Damjanova, I.; Puskás, E.; Jánvári, L.; Farkas, M.; Dobák, A.; Böröcz, K.; Pászti, J. Emergence of a colistin-resistant KPC-2-producing Klebsiella pneumoniae ST258 clone in Hungary. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Kontopoulou, K.; Protonotariou, E.; Vasilakos, K.; Kriti, M.; Koteli, A.; Antoniadou, E.; Sofianou, D. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J. Hosp. Infect. 2010, 76, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Neonakis, I.K.; Samonis, G.; Messaritakis, H.; Baritaki, S.; Georgiladakis, A.; Maraki, S.; Spandidos, D.A. Resistance status and evolution trends of Klebsiella pneumoniae isolates in a university hospital in Greece: Ineffectiveness of carbapenems and increasing resistance to colistin. Chemotherapy 2010, 56, 448–452. [Google Scholar] [CrossRef]
- Mezzatesta, M.L.; Gona, F.; Caio, C.; Petrolito, V.; Sciortino, D.; Sciacca, A.; Santangelo, C.; Stefani, S. Outbreak of KPC-3-producing, and colistin-resistant, Klebsiella pneumoniae infections in two Sicilian hospitals. Clin. Microbiol. Infect. 2011, 17, 1444–1447. [Google Scholar] [CrossRef] [Green Version]
- Mammina, C.; Bonura, C.; Di Bernardo, F.; Aleo, A.; Fasciana, T.; Sodano, C.; Saporito, M.A.; Verde, M.S.; Tetamo, R.; Palma, D.M. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Eurosurveillance 2011, 17, 20248. [Google Scholar] [CrossRef]
- Capone, A.; Giannella, M.; Fortini, D.; Giordano, A.; Meledandri, M.; Ballardini, M.; Venditti, M.; Bordi, E.; Capozzi, D.; Balice, M.P.; et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 2013, 19, E23–E30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Bliziotis, I.A. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO’s First Global Report on Antibiotic Resistance Reveals Serious, Worldwide Threat to Public Health. Available online: http://www.who.int/mediacentre/news/releases/2014/amr-report/en/ (accessed on 15 August 2019).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zerbaxa (accessed on 15 August 2019).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zavicefta (accessed on 15 August 2019).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaborem (accessed on 15 August 2019).
- Fraile-Ribot, P.A.; Mulet, X.; Cabot, G.; Del Barrio-Tofiño, E.; Juan, C.; Pérez, J.L.; Oliver, A. In Vivo Emergence of Resistance to Novel Cephalosporin-β-Lactamase Inhibitor Combinations through the Duplication of Amino Acid D149 from OXA-2 β-Lactamase (OXA-539) in Sequence Type 235 Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01117. [Google Scholar] [CrossRef] [PubMed]
- Fraile-Ribot, P.A.; Cabot, G.; Mulet, X.; Periañez, L.; Martín-Pena, M.L.; Juan, C.; Pérez, J.L.; Oliver, A. Mechanisms leading to in vivo ceftolozane/tazobactam resistance development during the treatment of infections caused by MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2018, 73, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; ECDC-EMA Working Group. Critical shortage of new antibiotics in development against multidrug-resistant bacteria-Time to react is now. Drug Resist. Updat. 2011, 14, 118–124. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Drug Approvals and Databases. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibiotic-treat-community-acquired-bacterial-pneumonia (accessed on 20 August 2019).
- Veve, M.P.; Wagner, J.L. Lefamulin: Review of a Promising Novel Pleuromutilin Antibiotic. Pharmacotherapy 2018, 38, 935–946. [Google Scholar] [CrossRef] [PubMed]



| Priority 1: Urgency “Critical” # |
| Acinetobacter baumannii, carbapenem-resistant |
| Pseudomonas aeruginosa, carbapenem-resistant § |
| Enterobacteriaceae *, carbapenem-resistant, 3rd generation cephalosporin-resistant |
| Priority 2: Urgency “High” |
| Enterococcus faecium, vancomycin-resistant |
| Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant |
| Helicobacter pylori, clarithromycin-resistant |
| Campylobacter spp., fluoroquinolone-resistant |
| Salmonella spp., fluoroquinolone-resistant |
| Neisseria gonorrhoeae, 3rd generation cephalosporin-resistant, fluoroquinolone-resistant |
| Priority 3: Urgency “Medium” |
| Streptococcus pneumoniae, penicillin-non-susceptible |
| Haemophilus influenzae, ampicillin-resistant |
| Shigella spp., fluoroquinolone-resistant |
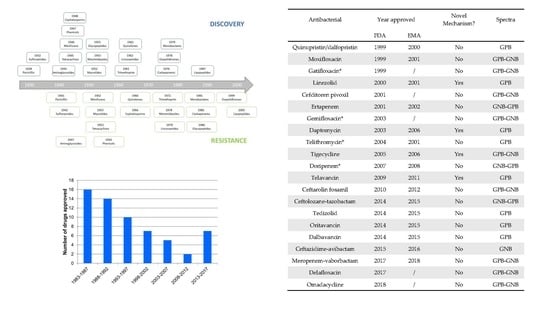
| Antibacterial | Year Approved | Novel Mechanism? | Spectra | |
|---|---|---|---|---|
| FDA | EMA | |||
| Quinupristin/dalfopristin | 1999 | 2000 | No | GPB |
| Moxifloxacin | 1999 | 2001 | No | GPB-GNB |
| Gatifloxacin * | 1999 | / | No | GPB-GNB |
| Linezolid | 2000 | 2001 | Yes | GPB |
| Cefditoren pivoxil | 2001 | / | No | GPB-GNB |
| Ertapenem | 2001 | 2002 | No | GNB-GPB |
| Gemifloxacin * | 2003 | / | No | GPB-GNB |
| Daptomycin | 2003 | 2006 | Yes | GPB |
| Telithromycin * | 2004 | 2001 | No | GPB |
| Tigecycline | 2005 | 2006 | Yes | GPB-GNB |
| Doripenem * | 2007 | 2008 | No | GNB-GPB |
| Telavancin | 2009 | 2011 | Yes | GPB |
| Ceftarolin fosamil | 2010 | 2012 | No | GPB-GNB |
| Ceftolozane-tazobactam | 2014 | 2015 | No | GNB-GPB |
| Tedizolid | 2014 | 2015 | No | GPB |
| Oritavancin | 2014 | 2015 | No | GPB |
| Dalbavancin | 2014 | 2015 | No | GPB |
| Ceftazidime-avibactam | 2015 | 2016 | No | GNB |
| Meropenem-vaborbactam | 2017 | 2018 | No | GPB-GNB |
| Delafloxacin | 2017 | / | No | GPB-GNB |
| Omadacycline | 2018 | / | No | GPB-GNB |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duval, R.E.; Grare, M.; Demoré, B. Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152. https://doi.org/10.3390/molecules24173152
Duval RE, Grare M, Demoré B. Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules. 2019; 24(17):3152. https://doi.org/10.3390/molecules24173152
Chicago/Turabian StyleDuval, Raphaël E., Marion Grare, and Béatrice Demoré. 2019. "Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria" Molecules 24, no. 17: 3152. https://doi.org/10.3390/molecules24173152
APA StyleDuval, R. E., Grare, M., & Demoré, B. (2019). Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules, 24(17), 3152. https://doi.org/10.3390/molecules24173152