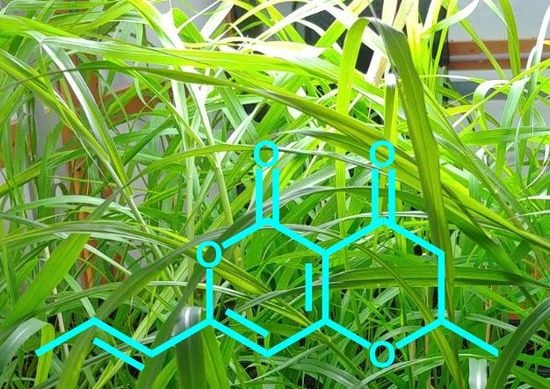
Synthesis and Herbicidal Activity Against Buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Synthesis of (±)-3-deoxyradicinin (2)
2.2. Phytotoxicity Assays on C. ciliaris
3. Materials and Methods
3.1. General Experimental Procedures
3.2. ((2,2-Dimethyl-4-methylene-4H-1,3-dioxin-6-yl)oxy)-trimethylsilane (4)
3.3. (E)-6-(2-hydroxypent-3-en-1-yl)-2,2-dimethyl-4H-1,3-dioxyn-4-one (5a)
3.4. 6-(2-Hydroxypropyl)-2,2-dimethyl-4H-1,3-dioxyn-4-one (5b)
3.5. (E)-2,2-dimethyl-6-(2-oxopent-3-en-1-yl)-4H-1,3-dioxyn-4-one (6a)
3.6. 2,2-Dimethyl-6-(2-oxopropyl)-4H-1,3-dioxyn-4-one (6b)
3.7. (E)-4-hydroxy-6-(propen-1-yl)-2H-piran-2-one (7a)
3.8. 4-Hydroxy-6-(methyl)-2H-piran-2-one (7b)
3.9. (E)-2-methyl-7-(prop-1-en-1-yl)-2,3-dihydropyrane[4,3-b]pyran-4,5-dione ((±)-3-deoxyradicinin; (±)-2)
3.10. 2,7-Dimethyl-2,3-dihydropyrane[4,3-b]pyran-4,5-dione ((±)-8)
3.11. Oxidation of (±)-3-deoxyradicinin (2) with Pb(OAc)4
3.12. Leaf Puncture Bioassays
3.13. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dayan, F.E.; Duke, S.O. Natural compounds as next generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Man. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Abella, S.R.; Chiquoine, L.P.; Backer, D.M. Ecological characteristics of sites invaded by buffelgrass (Pennisetum ciliare). Invasive Plant. Sci. Manag. 2012, 5, 443–453. [Google Scholar] [CrossRef]
- Bean, T.M. Tools for Improved Management of Buffelgrass in the Sonoran Desert. Ph.D. Thesis, The University of Arizona, Phoenix, AZ, USA, 2014. [Google Scholar]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Cristofaro, M.; Evidente, A. Cochliotoxin, a dihydropyranopyran-4,5-dione, and its analogues produced by Cochliobolus australiensis display phytotoxic activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Evidente, A. Chloromonilinic acids C and D, two phytotoxic tetrasubstituted 3-chromanonacrylic acids isolated from Cochliobolus australiensis with potential herbicidal activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 2771–2777. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Górecki, M.; Mandoli, A.; Di Bari, L.; Pescitelli, G.; Cimmino, A.; Cristofaro, A.; Clement, S.; Evidente, A. Pyriculins A and B, two monosubstituted hex-4-ene-2,3-diols and other phytotoxic metabolites produced by Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2017, 29, 726–736. [Google Scholar] [CrossRef]
- Clarke, D.D.; Nord, F.F. Radicinin: A new pigment from Stemphylium radicinum. Arch. Biochem. Biophys. 1953, 45, 469–470. [Google Scholar] [CrossRef]
- Grove, J.F. Metabolic products of Stemphylium radicinum. Part I. Radicinin. J. Chem. Soc. 1964, 3234–3239. [Google Scholar] [CrossRef]
- Robeson, D.J.; Gray, G.R.; Strobel, G.A. Production of the phytotoxins radicinin and radicinol by Alternaria chrysanthemi. Phytochemistry 1982, 21, 2359–2362. [Google Scholar] [CrossRef]
- Nukina, M.; Marumo, S. Radicinol, a new metabolite of Cochliobolus lunata, and absolute stereochemistry of radicinin. Tetrahedron Lett. 1977, 37, 3271–3272. [Google Scholar] [CrossRef]
- Tal, B.; Robeson, D.J.; Burke, B.A.; Aasen, A.J. Phytotoxins from Alternaria helianti: Radicinin and the structures of deoxyradicinol and radianthin. Phytochemistry 1985, 24, 729–731. [Google Scholar] [CrossRef]
- Noordeloos, M.E.; De Gruyter, J.; Van Eijl, G.W.; Roeijmans, H.J. Production of dendritic crystals in pure cultures of Phoma and Ascochyta and its value as a taxonomic character relative to morphology, pathology and cultural characteristics. Micol. Res. 1993, 97, 1343–1350. [Google Scholar] [CrossRef]
- Kadam, S.; Poddig, J.; Humphrey, P.; Karwowski, J.; Jackson, M.; Tennent, S.; Fung, T.; Hochlowski, J.; Rasmussen, R.; McAlpine, J. Citrine hydrate and radicinin: Uman rinovirus 3-C protease inhibitors discovered in target-directed microbial screen. J. Antib. 1994, 47, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Ishida, T.; Otsuka, Y.; Hamasaki, T.; Ichinoe, M. Phytotoxins and related metabolites produced by Bipolaris coicis, the pathogen of job′s tears. Phytochemistry 1997, 45, 41–45. [Google Scholar] [CrossRef]
- Pryor, B.M.; Gilbertson, R.L. Relationships and taxonomic status of Alternaria radicina, A. carotincultae, and A. petroselini based upon morphological, biochemical, and molecular characteristics. Mycologia 2002, 94, 49–61. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabrò, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shao, M.W.; Lu, Y.H.; Kong, L.C.; Jiang, D.H.; Zhang, Y.L. Phytotoxic and antibacterial metabolites from Fusarium proliferatum ZS07 isolated from the gut of long-horned grasshoppers. J. Agric. Food Chem. 2014, 62, 8997–9001. [Google Scholar] [CrossRef]
- Hussain, H.; Kliche-Spory, C.; Al-Harrasi, A.; Al-Rawahi, A.; Abbas, G.; Green, I.R.; Schulz, B.; Krohn, K.; Shah, A. Antimicrobial constituents from three endophytic fungi. Asian Pac. J. Trop. Med. 2014, 7, S224–S227. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce′s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Evidente, A. Effect of cultural conditions on the production of radicinin, a specific fungal phytotoxin for buffelgrass (Cenchrus ciliaris) biocontrol, by different Cochlioboulus australiensis strains. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manan, M.; Webb, C. Estimating fungal growth in submerged fermentation in the presence of solid particles based on colour development. Biotech. Biotechnol. Equipm. 2018, 32, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Sakuno, E.; Ishihara, A.; Tamura, J.I.; Nakajima, H. Conversions of deoxyradicinin to radicinin and of radicinin to 3-epi-radicinin in the phytopathogenic fungus Bipolaris coicis. Phytochemistry 2012, 75, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Freda, F.; Clement, S.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Phytotoxic activity and structure–activity relationships of radicinin derivatives against the invasive weed buffelgrass (Cenchrus ciliaris). Molecules 2019, 24, 2793. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Hirata, Y.; Yamamura, S. Syntheses of (±)-radicinin and (±)-dihydroradicinin. J. Chem. Soc. 1969, 15, 1997–2002. [Google Scholar] [CrossRef]
- Yamamura, S.; Kato, K.; Hirata, Y. The reaction of 6-carboxymethyl-4-methoxy-2-pyrone with acetic anhydride. Chem. Comm. 1968, 1580. [Google Scholar] [CrossRef]
- Grunwell, J.R.; Karipides, A.; Wigal, J.C.; Heinzman, S.V.; Parlow, J.; Surso, J.A.; Claytone, L.; Fleitz, F.J.; Daffner, M.; Stevens, J.E. The formal oxidative addition of electron-rich transoid dienes to bromonaphthoquinones. J. Org. Chem. 1991, 56, 91–95. [Google Scholar] [CrossRef]
- Bach, T.; Kirsch, S. Synthesis of 6-substituted 4-hydroxy-2-pyrones from aldehydes by addition of an acetoacetate equivalent, Dess–Martin oxidation and subsequent cyclization. Synlett 2001, 1974–1976. [Google Scholar] [CrossRef]
- Fang, Z.; Clarkson, G.J.; Wills, M. Asymmetric reduction of 2,2-dimethyl-6-(2-oxoalkyl/oxoaryl)-1,3-dioxin-4-ones and application to the synthesis of (+)-yashabushitriol. Tetrahedron Lett. 2013, 54, 6834–6837. [Google Scholar] [CrossRef] [Green Version]
- Demuner, A.J.; Moreira Valente, V.M.; Almeida Barbosa, L.C.; Rathi, A.H.; Donohoe, T.J.; Thompson, A.L. Synthesis and phytotoxic activity of new pyridones derived from 4-hydroxy-6-methylpyridin-2(1H)-one. Molecules 2009, 14, 4973–4986. [Google Scholar] [CrossRef]
- Zehnder, L.R.; Dahly, J.W.; Hsung, R.P. Lewis acid mediated condensation reactions of α,β-unsaturated acids with 4-hydroxy-2-pyrones. A concise structural assignment of Fleischmann′s α,α-bispyrone and Praill’s α,γ-bispyrone. Tetrahedron Lett. 2000, 41, 1901–1905. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (±)-2, (±)-8, and 10 are available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsico, G.; Ciccone, M.S.; Masi, M.; Freda, F.; Cristofaro, M.; Evidente, A.; Superchi, S.; Scafato, P. Synthesis and Herbicidal Activity Against Buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules 2019, 24, 3193. https://doi.org/10.3390/molecules24173193
Marsico G, Ciccone MS, Masi M, Freda F, Cristofaro M, Evidente A, Superchi S, Scafato P. Synthesis and Herbicidal Activity Against Buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules. 2019; 24(17):3193. https://doi.org/10.3390/molecules24173193
Chicago/Turabian StyleMarsico, Giulia, Maria Sabrina Ciccone, Marco Masi, Fabrizio Freda, Massimo Cristofaro, Antonio Evidente, Stefano Superchi, and Patrizia Scafato. 2019. "Synthesis and Herbicidal Activity Against Buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin" Molecules 24, no. 17: 3193. https://doi.org/10.3390/molecules24173193
APA StyleMarsico, G., Ciccone, M. S., Masi, M., Freda, F., Cristofaro, M., Evidente, A., Superchi, S., & Scafato, P. (2019). Synthesis and Herbicidal Activity Against Buffelgrass (Cenchrus ciliaris) of (±)-3-deoxyradicinin. Molecules, 24(17), 3193. https://doi.org/10.3390/molecules24173193