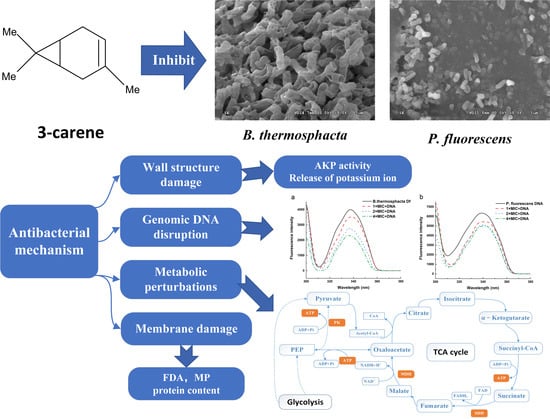
Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens
Abstract
:1. Introduction
2. Results
2.1. Inhibition Effect of 3-Carene on B. thermosphacta and P. fluorescens Strains
2.2. Cell Wall Permeability of B. thermosphacta and P. fluorescens
2.3. Release of Potassium Ion
2.4. The Effect of 3-Carene on Inhibiting Cell Membrane of B. thermosphacta and P. fluorescens
2.5. Effect of 3-Carene on the Cell Ultrastructure of B. thermosphacta and P. fluorescens
2.6. Protein Synthesis
2.7. Effect of 3-Carene on Bacterial Membrane Potential (MP)
2.8. Effect of 3-Carene on Enzyme Activity
2.9. Effect of 3-Carene on the Bacterial ATP Concentration
2.10. Effect of 3-Carene on B. thermosphacta and P. fluorescens DNA
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Chemicals
4.2. Determination of MIC
4.3. The Inhibition Effect of 3-Carene on the Growth Curve
4.4. Determination of Cell Wall Integrity
4.5. Potassium Ion Release Assay
4.6. Fluorescein Diacetate Staining Experiment
4.7. Scanning Electron Microscopy (SEM) Observation
4.8. Membrane Potential (MP) Determinations
4.9. Protein Content, and SDH, MDH, and PK Activities
4.10. Measurement of Intracellular ATP Concentrations
4.11. The Effect of 3-Carene on Bacterial Genomic DNA
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huis in’t Veld, J.H.J. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Zhao, G.-P.; Li, Y.-Q.; Sun, G.-J.; Mo, H.-Z. Antibacterial actions of glycinin basic peptide against Escherichia coli. J. Agric. Food Chem. 2017, 65, 5173–5180. [Google Scholar] [CrossRef] [PubMed]
- Illikoud, N.; Jaffrès, E.; Zagorec, M. Brochothrix thermosphacta. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Jiang, Y.; Gao, F.; Xing-Lian, X.U.; Ke-Ping, Y.E.; Zhou, G.H. Microfloral change of vacuum-packaged pork during chilled storage. Food Sci. 2011, 32, 241–245. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Taxonomy, role, and significance of microorganisms in foods. In Modern Food Microbiology; Springer: Boston, MA, USA, 1992; pp. 13–37. [Google Scholar]
- Ray, B.; Bhunia, A. Fundamental Food Microbiology; CRC Press: Boca Raton, FL, USA, 2004; Volume 64, p. 33. [Google Scholar]
- Olanya, O.M.; Ukuku, D.O.; Niemira, B.A. Effects of temperatures and storage time on resting populations of Escherichia coli O157:H7 and Pseudomonas fluorescens in vitro. Food Control. 2014, 39, 128–134. [Google Scholar] [CrossRef]
- Meyer, A.; Suhr, K.; Nielsen, P.; Holm, F. Natural food preservatives. In Minimal Processing Technologies in the Food Industries; Woodhead Publishing Limited and CRC Press: Boca Raton, FL, USA, 2002; pp. 124–174. [Google Scholar]
- Liu, H.; Pei, H.; Han, Z.; Feng, G.; Li, D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control. 2015, 47, 444–450. [Google Scholar] [CrossRef]
- Fleming-Jones, M.E.; Smith, R.E. Volatile organic compounds in foods: A five year study. J. Agric. Food Chem. 2003, 51, 8120–8127. [Google Scholar] [CrossRef]
- Bonilla, J.; Vargas, M.; Atarés, L.; Chiralt, A. Effect of chitosan essential oil films on the storage-keeping quality of pork meat products. Food Bioprocess. Technol. 2014, 7, 2443–2450. [Google Scholar] [CrossRef]
- Chen, Z.; He, B.; Zhou, J.; He, D.; Deng, J.; Zeng, R. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Ind. Crop. Prod. 2016, 83, 607–613. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control. 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Zou, L.; Hu, Y.-Y.; Chen, W.-X. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015, 52, 8196–8203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Chen, W.; Dou, Z.-M.; Chen, R.; Hu, Y.; Chen, W.; Chen, H. Antimicrobial effect of black pepper petroleum ether extract for the morphology of Listeria monocytogenes and Salmonella typhimurium. J. Food Sci. Technol. 2017, 54, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mancini, E.; de Almeida, L.F.R.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Musenga, A.; Mandrioli, R.; Ferranti, A.; D’Orazio, G.; Fanali, S.; Raggi, M.A. Analysis of aromatic and terpenic constituents of pepper extracts by capillary electrochromatography. J. Sep. Sci. 2007, 30, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Balamayooran, G.; Batra, S.; Fessler, M.B.; Happel, K.I.; Jeyaseelan, S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Respir. Cell Mol. Biol. 2010, 43, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Štefková, K.; Procházková, J.; Pacherník, J. Alkaline phosphatase in stem cells. Stem. Cells Int. 2015, 2015, 628368. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Codling, C.E.; Maillard, J.-Y.; Russell, A.D. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J. Antimicrob. Chemother. 2003, 51, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Young, D.H.; Köhle, H.; Kauss, H. Effect of chitosan on membrane permeability of suspension-cultured glycine max and phaseolus vulgaris cells. Plant. Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970, 45, 115–120. [Google Scholar] [CrossRef]
- Widholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Chowdhury, S.R.; Mukherjea, K.K. DNA binding and nuclease activity of a one-dimensional heterometallic nitrosyl complex. IntJ. Biol. Macromol. 2007, 41, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-L.; Shi, Y.-H.; Zhao, W.; Hao, G.; Le, G.-W. Interaction of MDpep9, a novel antimicrobial peptide from Chinese traditional edible larvae of housefly, with Escherichia coli genomic DNA. Food Chem. 2009, 115, 867–872. [Google Scholar] [CrossRef]
- Magalhães, L.G.; Souza, J.M.D.; Wakabayashi, K.A.L.; Laurentiz, R.D.S.; Vinhólis, A.H.C.; Rezende, K.C.S.; Simaro, G.V.; Bastos, J.K.; Rodrigues, V.; Esperandim, V.R. In vitro efficacy of the essential oil of Piper cubeba L. (Piperaceae) against Schistosoma mansoni. Parasitol Res. 2012, 110, 1747–1754. [Google Scholar]
- Durand, E.; Lecomte, J.M.; Villeneuve, P. The biological and antimicrobial activities of phenolipids. Lipid Technol. 2017, 29, 67–70. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, X.; Pan, S.; Fang, Y.; Jiang, F.; Phillips, G.O.; Xu, X. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012, 132, 1883–1890. [Google Scholar] [CrossRef]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 286–292. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Drider, D.; Kheadr, E.; Lacroix, C.; Prévost, H.; Fliss, I. Multiple characterizations of Listeria monocytogenes sensitive and insensitive variants to divergicin M35, a new pediocin-like bacteriocin. J. Appl. Microbiol 2010, 100, 29–39. [Google Scholar] [CrossRef]
- Doughari, J.H.; Ndakidemi, P.A.; Human, I.S.; Benade, S. Antioxidant, antimicrobial and antiverotoxic potentials of extracts of Curtisia dentata. J. Ethnopharmacol. 2012, 141, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.C.; Li, H.T.; Du, G.C.; Guo, D.S. A study on the antibacterial mechanism of rosmarinic acid. J. Qingdao Univ. (Nat. Sci.) 2005, 18, 41–45. [Google Scholar]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Mitchell, P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim. Biophys. Acta 2011, 1807, 1507–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novo, D.; Perlmutter, N.G.; Hunt, R.H.; Shapiro, H.M. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 1999, 35, 55–63. [Google Scholar] [CrossRef]
- Rodríguez, E.; Seguer, J.; Rocabayera, X.; Manresa, A. Cellular effects of monohydrochloride of L-arginine, N-lauroyl ethylester (LAE) on exposure to Salmonella typhimurium and Staphylococcus aureus. J. Appl. Microbiol 2010, 96, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant. Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Pantev, A.; Valcheva, R.; Danova, S.; Ivanova, I.; Minkov, I.; Haertlé, T.; Chobert, J.M. Effect of enterococcin A 2000 on biological and synthetic phospholipid membranes. Int. J. Food Microbiol. 2003, 80, 145–152. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M. Citric acid cycle and role of its Intermediates in Metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Ming-Sheng, X.U.; Chen, J.P.; Shangguan, X.C. Effects of protamine on succinate dehydrogenase and malata dehydrogenase in cells of aspergillus niger. Food Sci. 2005. [Google Scholar]
- Boyd, E.F.; Nelson, K.; Wang, F.S.; Whittam, T.S.; Selander, R.K. Molecular genetic basis of allelic polymorphism in malate dehydrogenase (mdh) in natural populations of Escherichia coli and Salmonella enterica. Proc. Natl. Acad. Sci. USA 1994, 91, 1280–1284. [Google Scholar] [CrossRef]
- Pilane, M.C.; Bagla, V.P.; Mokgotho, M.P.; Mbazima, V.; Matsebatlela, T.M.; Ncube, I.; Mampuru, L. Free radical scavenging activity: Antiproliferative and proteomics analyses of the differential expression of apoptotic proteins in mcf-7 cells treated with acetone leaf extract of diospyros lycioides (ebenaceae). Evid. Based Complement. Altern. Med. 2015, 2015, 534808. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Kets, E.P.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Env. Microbiol 1999, 65, 4606–4610. [Google Scholar]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Mori, A.; Nishino, C.; Enoki, N.; Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Venkatesalu, V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004, 91, 105–108. [Google Scholar] [CrossRef]
- Babii, C.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol 2016, 120, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Tang, Z.X.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Foods 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Hao, G.; Shi, Y.-H.; Tang, Y.-L.; Le, G.-W. The membrane action mechanism of analogs of the antimicrobial peptide Buforin 2. Peptides 2009, 30, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Env. Microbiol. 1982, 43, 1256–1261. [Google Scholar]
- Cooper, R.A. Inhibition of biofilms by glucose oxidase, lactoperoxidase and guaiacol: The active antibacterial component in an enzyme alginogel. Int. WoundJ. 2013, 10, 630–637. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Marie, D.; Vaulot, D.; Partensky, F. Application of the novel nucleic acid dyes YOYO-1, YO-PRO-1, and PicoGreen for flow cytometric analysis of marine prokaryotes. Appl. Env. Microbiol. 1996, 62, 1649–1655. [Google Scholar] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sánchez, E.; García, S.; Heredia, N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Env. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |










| Bacteria | Control | The Concentration of 3-Carene (mL/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | 2% Ethanol | 0.625 | 1.25 | 2.5 | 5 | 10 | 20 | 40 | |
| B.thermosphacta | +++ | +++ | +++ | +++ | +++ | ++ | + | − | − |
| P. fluorescens | +++ | +++ | +++ | +++ | +++ | +++ | ++ | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. https://doi.org/10.3390/molecules24183246
Shu H, Chen H, Wang X, Hu Y, Yun Y, Zhong Q, Chen W, Chen W. Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules. 2019; 24(18):3246. https://doi.org/10.3390/molecules24183246
Chicago/Turabian StyleShu, Huizhen, Haiming Chen, Xiaolong Wang, Yueying Hu, Yonghuan Yun, Qiuping Zhong, Weijun Chen, and Wenxue Chen. 2019. "Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens" Molecules 24, no. 18: 3246. https://doi.org/10.3390/molecules24183246
APA StyleShu, H., Chen, H., Wang, X., Hu, Y., Yun, Y., Zhong, Q., Chen, W., & Chen, W. (2019). Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules, 24(18), 3246. https://doi.org/10.3390/molecules24183246