Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Single-Factor Experimental Extraction Conditions
2.1.1. Effects of Ultrasonic Temperature on TFC
2.1.2. Effects of Ultrasonic Time on TFC
2.1.3. Effects of Ethanol Concentration on TFC
2.1.4. Effects of Liquid–Solid Ratio on TFC
2.2. Model Fitting
2.3. Effects of the Variables on TFC
2.4. Effects of the Variables on Antioxidant Activity
2.4.1. Effects of the Variables on DPPH-RSC
2.4.2. Effects of the Variables on FRAP
2.5. Optimization Extraction Conditions and Verification of Predictive Model
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Equipment for UAE
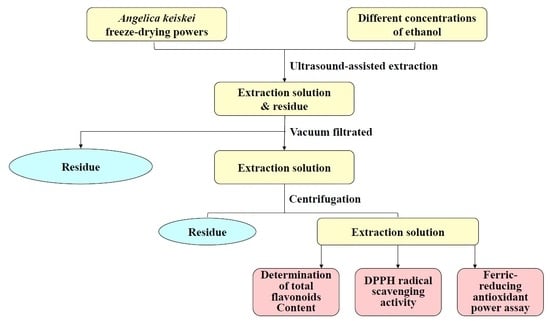
3.4. UAE of Flavonoids from A. keiskei
3.5. Single-Factor Experiments
3.6. BBD for Extraction Optimization
3.7. Determination of Total Flavonoid Content (TFC)
3.8. Determination of Antioxidant Activities
3.8.1. DPPH Radical Scavenging Capacity (DPPH-RSC) Measurement
3.8.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Liu, D.; Gao, H. Kinetics, physicochemical properties, and antioxidant activities of Angelica keiskei processed under four drying conditions. LWT Food Sci. Technol. 2018, 98, 349–357. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Zimmermann, A.; Kainz, K.; Pietrocola, F.; Chen, G.; Maglioni, S.; Schiavi, A.; Nah, J.; Mertel, S.; Beuschel, C.B.; et al. The flavonoid 4,4’-dimethoxychalcone promotes autophagy-dependent longevity across species. Nat. Commun. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.S.; Choi, S.K.; Lee, Y.S.; Jafari, M.; Seo, E.K. Chalcones from Angelica keiskei: Evaluation of Their Heat Shock Protein Inducing Activities. J. Nat. Prod. 2015, 78, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Wang, L.; Gao, X.; Li, Q. Research Progress of Anti-tumor Effects of Chalcones from Angelica keiskei. Sci. Technol. Food Ind. 2018, 39, 336–352. [Google Scholar] [CrossRef]
- Nakamura, T.; Tokushima, T.; Kawabata, K.; Yamamoto, N.; Miyamoto, M.; Ashida, H. Absorption and metabolism of 4-hydroxyderricin and xanthoangelol after oral administration of Angelica keiskei (Ashitaba) extract in mice. Arch. Biochem. Biophys. 2012, 521, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Gao, L.X.; Meng, F.W.; Tang, C.L.; Zhang, R.J.; Li, J.Y.; Luo, C.; Li, J.; Zhao, W.M. PTP1B inhibitors from stems of Angelica keiskei (Ashitaba). Bioorg. Med. Chem. Lett. 2015, 25, 2028–2032. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Q.; Luo, J.; Wu, J.; Wang, Z. Phytonutrient and anti-diabetic functional properties of flavonoid-rich ethanol extract from Angelica keiskei leaves. J. Food Sci. Technol. 2018, 55, 4406–4412. [Google Scholar] [CrossRef]
- Xie, F.; Wang, Y.; Zhou, Y.; Wu, J.; Wang, Z. Effect of lactic acid bacteria on microbial safety of angelica keiskei juice. J. Food Saf. 2017, 37, e12325. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Natural medicine: The genus Angelica. Curr. Med. Chem. 2004, 11, 1479–1500. [Google Scholar] [CrossRef]
- Zhang, T.; Yamashita, Y.; Yasuda, M.; Yamamoto, N.; Ashida, H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funct. 2015, 6, 135–145. [Google Scholar] [CrossRef]
- Nikitakis, J.; Lange, B. International Cosmetic Ingredient Dictionary and Handbook, 6th ed.; Personal Care Products Council: Washington, DC, USA, 2016. [Google Scholar]
- Chen, C.Y. Trace elements in Taiwanese health food, Angelica keiskei, and other products. Food Chem. 2004, 84, 545–549. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, M.; Mujumdar, A.S.; Wang, Z. Effect of microwave freeze-drying on microbial inactivation, antioxidant substance and flavor quality of Ashitaba leaves (Angelica keiskei Koidzumi). Dry. Technol. 2019, 37, 793–800. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsusita, Y.I.; Matsui, K.; Kurogi, C.; Matsui, T. Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 2011, 67, 5346–5359. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, H.J.; Kim, Y.M.; Park, S.J.; Rho, M.C.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Characteristic of alkylated chalcones from Angelica keiskei on influenza virus neuraminidase inhibition. Bioorg. Med. Chem. Lett. 2011, 21, 5602–5604. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Nakata, K.; Miyazaki, H.; Maehashi, S.; Komiyama, Y.; Aida, R.; Yoshida, S.; Kokubu, D.; Hagiwara, K.; Yoshida, K. 4-Hydroxyderricin inhibits osteoclast formation and accelerates osteoblast differentiation. Cytotechnology 2019, 71, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Gao, M.; Han, M.; Liu, K.; Zhang, Z.; Gong, Z.; Xing, L.; Shi, X.; Lu, K.; et al. Endoplasmic reticulum stress triggers Xanthoangelol-induced protective autophagy via activation of JNK/c-Jun Axis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 8. [Google Scholar] [CrossRef]
- Ogawa, H.; Ohno, M.; Baba, K. Hypotensive and lipid regulatory actions of 4-hydroxyderricin, a chalcone from Angelica keiskei, in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2005, 32, 19–23. [Google Scholar] [CrossRef]
- Yasuda, M.; Kawabata, K.; Miyashita, M.; Okumura, M.; Yamamoto, N.; Takahashi, M.; Ashida, H.; Ohigashi, H. Inhibitory effects of 4-hydroxyderricin and xanthoangelol on lipopolysaccharide-induced inflammatory responses in RAW264 macrophages. J. Agric. Food Chem. 2014, 62, 462–467. [Google Scholar] [CrossRef]
- Ohnogi, H.; Kudo, Y.; Tahara, K.; Sugiyama, K.; Enoki, T.; Hayami, S.; Sagawa, H.; Tanimura, Y.; Aoi, W.; Naito, Y.; et al. Six new chalcones from Angelica keiskei inducing adiponectin production in 3T3-L1 adipocytes. Biosci. Biotechnol. Biochem. 2012, 76, 961–966. [Google Scholar] [CrossRef]
- Kil, Y.S.; Pham, S.T.; Seo, E.K.; Jafari, M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch. Pharmacal Res. 2017, 40, 655–675. [Google Scholar] [CrossRef]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The Antioxidant Activity of Coumarins and Flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.; Shi, J.; Tomas-Barberan, F.; Datta, N.; Singanusong, R.; Chen, S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Oludemi, T.; Barros, L.; Prieto, M.A.; Heleno, S.A.; Barreiro, M.F.; Ferreira, I. Extraction of triterpenoids and phenolic compounds from Ganoderma lucidum: Optimization study using the response surface methodology. Food Funct. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, R.; Guo, Y.; He, J.; Li, S.; Zhu, Z.; Liu, G.; Liu, Z.; Yang, J. Optimization of ultrasound-assisted extraction of gardenia fruit oil with bioactive components and their identification and quantification by HPLC-DAD/ESI-MS2. Food Funct. 2015, 6, 2194–2204. [Google Scholar] [CrossRef]
- Farrell, A.E.; Plevin, R.J.; Turner, B.T.; Jones, A.D.; Michael, O.H.; Kammen, D.M. Ethanol can contribute to energy and environmental goals. Science 2006, 311, 506–508. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B (Methodol.) 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuna, C.; Canas-Sarazua, R. Optimization of extraction yield, flavonoids and lycopene from tomato pulp by high hydrostatic pressure-assisted extraction. Food Chem. 2019, 278, 751–759. [Google Scholar] [CrossRef]
- Agarwal, C.; Mathe, K.; Hofmann, T.; Csoka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa L. Optimized by Response Surface Methodology. J. Food Sci. Technol. 2018, 83, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Li, Q.; Jin, W.; Chen, W.; Han, J.; Zhang, Y. Simultaneous Optimization for Ultrasound-Assisted Extraction and Antioxidant Activity of Flavonoids from Sophora flavescens Using Response Surface Methodology. Molecules 2018, 24, 112. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.X.; Zou, L.; Zhao, J.L.; Xiang, D.B.; Zhu, P.; Zhao, G. Response surface modeling and optimization of ultrasound-assisted extraction of three flavonoids from tartary buckwheat (Fagopyrum tataricum). Pharmacogn. Mag. 2013, 9, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lu, T.; Wang, M.; Zou, X.; Zhang, Y.; Yang, X.; Dong, Y.; Zhou, H. Flavonoids from Morus alba L. Leaves: Optimization of Extraction by Response Surface Methodology and Comprehensive Evaluation of Their Antioxidant, Antimicrobial, and Inhibition of alpha-Amylase Activities through Analytical Hierarchy Process. Molecules 2019, 24, 2398. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.L.; Wan, P.F.; Dong, C.L.; Li, Y.H. Optimization of total flavonoids content extracted from Flos Populi using response surface methodology. Ind. Crop. Prod. 2013, 43, 778–786. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Wang, H.; Wang, D.; Fang, F.; Wang, F.; Wu, T. Ultrasonic extraction of antioxidants from Chinese sumac (Rhus typhina L.) fruit using response surface methodology and their characterization. Molecules 2014, 19, 9019–9032. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Karoglan Kontić, J. Multi-response optimisation of ultrasound-assisted extraction for recovery of flavonoids from red grape skins using response surface methodology. Phytochem. Anal. 2016, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Muhammad, S.; Chen, A.; Chen, P.; Wang, J.; Yang, C.; Yuan, H.; Wang, Z. An experimental study exploring the influencing factors for ultrasonic-assisted extraction of flavonoid compounds from leaves of Amorpha fruticosa L. J. For. Res. 2019, 30, 1735–1741. [Google Scholar] [CrossRef]
- Zhang, Y.; Prawang, P.; Li, C.; Meng, X.; Zhao, Y.; Wang, H.; Zhang, S. Ultrasonic assisted extraction of artemisinin from Artemisia Annua L. using monoether-based solvents. Green Chem. 2018, 20, 713–723. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mao, Y.D.; Wang, Y.F.; Raza, A.; Qiu, L.P.; Xu, X.Q. Optimization of Ultrasonic-Assisted Enzymatic Extraction Conditions for Improving Total Phenolic Content, Antioxidant and Antitumor Activities in Vitro from Trapa quadrispinosa Roxb. Residues. Molecules 2017, 22, 396. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.P.; Zhang, H.S.; Huang, Q. Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohydr. Polym. 2012, 87, 614–619. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. Optimization of Drying of Olive Leaves in a Pilot-Scale Heat Pump Dryer. Dry. Technol. 2009, 27, 416–427. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Srinivasakannan, C.; Peng, J. Application of response surface methodology for optimization of the synthesis of synthetic rutile from titania slag. Appl. Surf. Sci. 2012, 258, 3068–3073. [Google Scholar] [CrossRef]
- Eslami, A.; Asadi, A.; Meserghani, M.; Bahrami, H. Optimization of sonochemical degradation of amoxicillin by sulfate radicals in aqueous solution using response surface methodology (RSM). J. Mol. Liq. 2016, 222, 739–744. [Google Scholar] [CrossRef]
- Kostić, M.D.; Bazargan, A.; Stamenković, O.S.; Veljković, V.B.; McKay, G. Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel 2016, 163, 304–313. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Zhu, C.P.; Zhai, X.C.; Li, L.Q.; Wu, X.X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Lin, T.; Liu, Y.; Lai, C.; Yang, T.; Xie, J.; Zhang, Y. The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind. Crop. Prod. 2018, 125, 150–159. [Google Scholar] [CrossRef]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC-ESI-DAD-MS. J. Food Sci. Technol. 2017, 54, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q. Response surface optimized ultrasonic-assisted extraction of flavonoids from Sparganii rhizoma and evaluation of their in vitro antioxidant activities. Molecules 2012, 17, 6769–6783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, L.; Hu, M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov. Food Sci. Emerg. Technol. 2011, 12, 18–25. [Google Scholar] [CrossRef]
- Wang, S.; Dong, X.; Tong, J. Optimization of enzyme-assisted extraction of polysaccharides from alfalfa and its antioxidant activity. Int. J. Biol. Macromol. 2013, 62, 387–396. [Google Scholar] [CrossRef]
- Impei, S.; Gismondi, A.; Canuti, L.; Canini, A. Metabolic and biological profile of autochthonous Vitis vinifera L. ecotypes. Food Funct. 2015, 6, 1526–1538. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Symbol | Response Variable | Fitting the Coding Equation of the Model |
|---|---|---|
| YTFC | Total flavonoid content (mg RE/g) | YTFC = 7.5459 + 0.0014X1 + 0.0529X2−0.2517X3 + 0.2763X4 − 0.1827X1X2 − 0.0065X1X3 + 0.0837X1X4 + 0.0992X2X3 − 0.3285X2X4 + 0.0278X3X4 − 0.0796X1² − 0.0099X2² − 0.5671X3² − 0.0266X4² |
| YDPPH | DPPH radical scavenging capacity (%) | YDPPH = 93.6219 − 0.2886X1 + 0.3209X2 + 1.4200X3 − 0.3100X4 − 0.8693X1X2 + 0.4834X1X3 + 0.6182X1X4 − 0.0165X2X3 − 0.5440X2X4 − 1.0414X3X4 + 0.0552X1² + 0.2049X2² + 0.0356X3² + 0.4082X4² |
| YFRAP | Ferric-reducing antioxidant power (μM Fe2+/g) | YFRAP = 49.6304 + 1.7734X1 + 1.6391X2 − 5.3144X3 + 2.2654X4 − 1.4726X1X2 + 1.8035X1X3 + 0.0422X1X4 + 0.5681X2X3 + 0.2908X2X4 + 1.3159X3X4 − 2.6215X1² − 1.6820X2² − 2.7125X3² − 3.1732X4² |
| Factor | Coefficient (β) | ||
|---|---|---|---|
| TFC | DPPH-RSC | FRAP | |
| Intercept | 7.5459 | 93.6219 | 49.6304 |
| Linear | |||
| X1 | 0.0014 | −0.2886 * | 1.7734 ** |
| X2 | 0.0529 | 0.3209 * | 1.6391 ** |
| X3 | −0.2517 *** | 1.4200 *** | −5.3144 *** |
| X4 | 0.2763 *** | −0.3100 * | 2.2654 *** |
| Cross product | |||
| X1X2 | −0.1827 * | −0.8693 *** | −1.4726 |
| X1X3 | −0.0065 | 0.4834 * | 1.8035 * |
| X1X4 | 0.0837 | 0.6182 * | 0.0422 |
| X2X3 | 0.0992 | −0.0165 | 0.5681 |
| X2X4 | −0.3285 *** | −0.5440 * | 0.2908 |
| X3X4 | 0.0278 | −1.0414 *** | 1.3159 |
| Quadratic | |||
| X1² | −0.0796 | 0.0552 | −2.6215 ** |
| X2² | −0.0099 | 0.2049 | −1.6820 * |
| X3² | −0.5671 *** | 0.0356 | −2.7125 *** |
| X4² | −0.0266 | 0.4082 * | −3.1732 *** |
| R² | 0.9417 | 0.9427 | 0.9413 |
| Adjusted R² | 0.8835 | 0.8854 | 0.8825 |
| Predicted R² | 0.7264 | 0.7106 | 0.7166 |
| Adequate precision | 16.3886 | 17.7803 | 14.0570 |
| Coefficient of variation | 1.95% | 0.4427% | 3.65% |
| p-Value (Model) | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| p-Value (Lack of fit) | 0.4747 | 0.2682 | 0.4000 |
| Response Variables | Optimum Extraction Conditions 1 | Maximum Value | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental Value 2 | Predicted Value | |
| YTFC (mg RE/g) | 80 °C | 4 min | 78% | 35 mL/g | 7.96 ± 0.18 | 8.29 |
| YDPPH (%) | 94.68 ± 0.57 | 95.25 | ||||
| YFRAP (μM Fe2+/g) | 45.35 ± 0.23 | 46.17 | ||||
| Independent Variable | Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ultrasonic temperature (°C) | X1 | 60 | 70 | 80 |
| Ultrasonic time (min) | X2 | 4 | 6 | 8 |
| Ethanol concentration (%) | X3 | 70 | 80 | 90 |
| Liquid-solid ratio (mL/g) | X4 | 25 | 30 | 35 |
| Run | Ultrasonic Temperature | Ultrasonic Time | Ethanol Concentration | Liquid-Solid Ratio | Response 1-YTFC | Response 2-YDPPH | Response 3-YFRAP |
|---|---|---|---|---|---|---|---|
| (℃) | (min) | (%) | (mL/g) | (mg RE/g) | (%) | (μM Fe2+/g) | |
| 1 | 60 | 6 | 70 | 30 | 6.95 | 93.09 | 47.97 |
| 2 | 80 | 8 | 80 | 30 | 7.27 | 92.88 | 45.92 |
| 3 | 70 | 6 | 80 | 30 | 7.52 | 93.29 | 48.20 |
| 4 | 60 | 6 | 80 | 35 | 7.73 | 93.55 | 45.93 |
| 5 | 80 | 6 | 70 | 30 | 7.00 | 91.49 | 49.38 |
| 6 | 60 | 8 | 80 | 30 | 7.74 | 94.92 | 45.66 |
| 7 | 80 | 6 | 80 | 35 | 7.96 | 94.00 | 48.43 |
| 8 | 60 | 6 | 80 | 25 | 7.14 | 95.25 | 41.82 |
| 9 | 70 | 4 | 70 | 30 | 7.36 | 92.15 | 50.65 |
| 10 | 80 | 6 | 80 | 25 | 7.04 | 93.22 | 44.15 |
| 11 | 70 | 6 | 80 | 30 | 7.43 | 93.36 | 48.06 |
| 12 | 60 | 4 | 80 | 30 | 7.34 | 93.36 | 40.36 |
| 13 | 70 | 4 | 80 | 25 | 6.79 | 93.47 | 39.37 |
| 14 | 80 | 4 | 80 | 30 | 7.61 | 94.79 | 46.51 |
| 15 | 70 | 8 | 80 | 25 | 7.62 | 95.70 | 42.96 |
| 16 | 60 | 6 | 90 | 30 | 6.68 | 94.92 | 34.54 |
| 17 | 70 | 6 | 70 | 35 | 7.47 | 93.16 | 49.11 |
| 18 | 70 | 6 | 80 | 30 | 7.68 | 93.80 | 51.47 |
| 19 | 70 | 6 | 90 | 35 | 6.93 | 94.53 | 40.19 |
| 20 | 70 | 4 | 80 | 35 | 7.93 | 93.81 | 44.95 |
| 21 | 70 | 6 | 80 | 30 | 7.69 | 94.06 | 50.24 |
| 22 | 70 | 6 | 70 | 25 | 7.10 | 91.73 | 48.50 |
| 23 | 70 | 8 | 70 | 30 | 7.28 | 93.14 | 52.82 |
| 24 | 70 | 8 | 90 | 30 | 6.84 | 95.38 | 43.45 |
| 25 | 70 | 6 | 80 | 30 | 7.41 | 93.60 | 50.17 |
| 26 | 80 | 6 | 90 | 30 | 6.71 | 95.25 | 43.16 |
| 27 | 70 | 6 | 90 | 25 | 6.45 | 97.26 | 34.32 |
| 28 | 70 | 4 | 90 | 30 | 6.52 | 94.46 | 39.01 |
| 29 | 70 | 8 | 80 | 35 | 7.44 | 93.87 | 49.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jiang, Y.; Pang, X.; Hua, P.; Gao, X.; Li, Q.; Li, Z. Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM). Molecules 2019, 24, 3461. https://doi.org/10.3390/molecules24193461
Zhang L, Jiang Y, Pang X, Hua P, Gao X, Li Q, Li Z. Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM). Molecules. 2019; 24(19):3461. https://doi.org/10.3390/molecules24193461
Chicago/Turabian StyleZhang, Lei, Yuhuan Jiang, Xuening Pang, Puyue Hua, Xiang Gao, Qun Li, and Zichao Li. 2019. "Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM)" Molecules 24, no. 19: 3461. https://doi.org/10.3390/molecules24193461
APA StyleZhang, L., Jiang, Y., Pang, X., Hua, P., Gao, X., Li, Q., & Li, Z. (2019). Simultaneous Optimization of Ultrasound-Assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM). Molecules, 24(19), 3461. https://doi.org/10.3390/molecules24193461