Matrix Discriminant Analysis Evidenced Surface-Lithium as an Important Factor to Increase the Hydrolytic Saccharification of Sugarcane Bagasse
Abstract
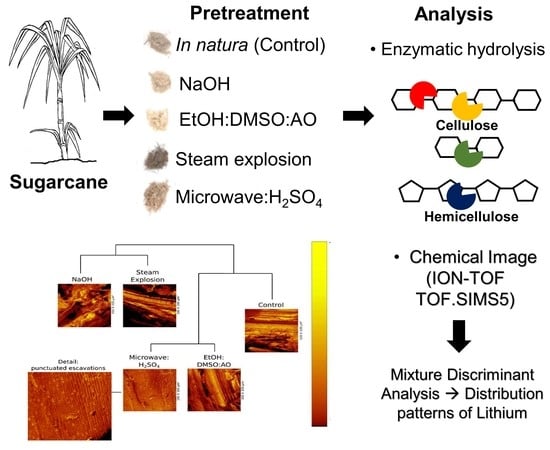
:1. Introduction
2. Results
2.1. Control-Milled Sugarcane Bagasse in Natura
2.2. Steam Explosion Pretreatment
2.3. Microwave:H2SO4 Pretreatment
2.4. Ethanol:Dimethyl Sulfoxide:Ammonium Oxalate (EtOH:DMSO:AO) Pretreatment
2.5. NaOH Pretreatment
2.6. Clustering Pretreatments Using Discriminant Analysis with Machine Learning—Anatomical Parameters
2.7. Clustering Pretreatments Using Discriminant Analysis with Machine Learning – Ionic Parameters
2.8. Performance of the Enzyme Cocktail Versus Pretreatment-the Enzymatic View Versus the Matrix Discriminant Analysis of Anatomical and Ionic Composition on the Surface of the Substrate
2.9. Matrix Discriminant Analysis (MDA) of Anatomical and Ionic Parameters from the Surface of the Substrate Versus the Enzymatic View—Correlation Between Discriminant Analysis and Saccharification Yields
2.10. Enzyme Cocktail Response to Metal Ion Salts in Solution
3. Discussion
4. Materials and Methods
4.1. Control—Milled Sugarcane Bagasse in Natura
4.2. Sugarcane Bagasse Pretreatments
4.2.1. Steam Explosion Pretreatment
4.2.2. Microwave:H2SO4 Pretreatment
4.2.3. Ethanol: Dimethyl Sulfoxide: Ammonium Oxalate Pretreatment
4.2.4. NaOH Pretreatment
4.3. Enzymatic Hydrolysis
4.4. Effects of Dissolved Salts on Specific Enzyme Activities
4.5. Chemical Image Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNICA. Avaliação Quinzenal da Safra 2018/2019 da Região Centro-Sul. 2018. Available online: http://www.unica.com.br/documentos/documentos/ (accessed on 15 December 2018).
- Rossetto, R.; Santiago, A.D. Cana-De-Açúcar. Available online: http://www.agencia.cnptia.embrapa.br/gestor/cana-de-acucar/arvore/CONTAG01_1_711200516715.html (accessed on 15 December 2018).
- Mohanram, S.; Amat, D.; Choudhary, J.; Arora, A.; Nain, L. Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustan. Chem. Process. 2013, 1, 1–15. [Google Scholar] [CrossRef]
- Goldbeck, R.; Damásio, A.R.L.; Gonçalves, T.A.; Machado, C.B.; Paixão, D.A.A.; Wolf, L.D.; Mandelli, F.; Rocha, G.J.M.; Ruller, R.; Squina, F.M. Development of hemicellulolytic enzyme mixtures for plant biomass deconstruction on target biotechnological applications. Appl. Microbiol. Biotechnol. 2014, 98, 8513–8525. [Google Scholar] [CrossRef] [PubMed]
- Buckeridge, M.S.; Grandis, A.; Tavares, E.Q. Disassembling the Glycomic Code of Sugarcane Cell Walls to Improve Second-Generation Bioethanol Production. In Bioethanol Production from Food Crops; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 31–43. [Google Scholar]
- Van Dyk, J.; Pletschke, B.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.Y.; Wu, S.; Fardim, P. Applications of ToF-SIMS in surface chemistry analysis of lignocellulosic biomass: A review. BioResources 2016, 11, 5581–5599. [Google Scholar]
- Purich, D.L. Enzyme Kinetics: Catalysis & Control: A reference of theory and best-practice methods, 1st ed.; Elsevier: London, UK, 2010; pp. 1–920. [Google Scholar]
- Segel, I.H. Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems, 1st ed.; John Wiley & Sons INC: New York, NY, USA, 1993; pp. 1–957. [Google Scholar]
- Shoseyov, O.; Shani, Z.; Levy, I. Carbohydrate Binding Modules: Biochemical Properties and Novel Applications. Microbiol. Mol. Boil. Rev. 2006, 70, 283–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, R.P.; Visser, J. Aspergillus Enzymes Involved in Degradation of Plant Cell Wall Polysaccharides. Microbiol. Mol. Boil. Rev. 2001, 65, 497–522. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Moretti, M.M.D.S.; Bocchini-Martins, D.A.; Nunes, C.D.C.C.; Villena, M.A.; Perrone, O.M.; Da Silva, R.; Boscolo, M.; Gomes, E. Pretreatment of sugarcane bagasse with microwaves irradiation and its effects on the structure and on enzymatic hydrolysis. Appl. Energy 2014, 122, 189–195. [Google Scholar] [CrossRef]
- Lima, M.S.; Damasio, A.R.D.L.; Crnkovic, P.M.; Pinto, M.R.; Da Silva, A.M.; Da Silva, J.C.R.; Segato, F.; De Lucas, R.C.; Jorge, J.A.; Polizeli, M.D.L.T.D.M. Co-cultivation of Aspergillus nidulans Recombinant Strains Produces an Enzymatic Cocktail as Alternative to Alkaline Sugarcane Bagasse Pretreatment. Front. Microbiol. 2016, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.F.; De Lucas, R.C.; Vitcosque, G.L.; Ribeiro, L.F.; Ward, R.J.; Rubio, M.V.; Damásio, A.R.; Squina, F.M.; Gregory, R.C.; Walton, P.H.; et al. A novel thermostable xylanase GH10 from Malbranchea pulchella expressed in Aspergillus nidulans with potential applications in biotechnology. Biotechnol. Biofuels 2014, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Segato, F.; Berto, G.L.; De Araújo, E.A.; Muniz, J.R.; Polikarpov, I. Expression, purification, crystallization and preliminary X-ray diffraction analysis of Aspergillus terreus endo-β-1,4-glucanase from glycoside hydrolase family 12. Acta Crystallogr. Sect. F Struct. Boil. Commun. 2014, 70, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Segato, F.; Damasio, A.R.L.; Gonçalves, T.A.; Murakami, M.T.; Squina, F.M.; Polizeli, M.; Mort, A.J.; Prade, R.A. Two structurally discrete GH7-cellobiohydrolases compete for the same cellulosic substrate fiber. Biotechnol. Biofuels 2012, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Szklarz, G.D.; Antibus, R.K.; Sinsabaugh, R.L.; Linkins, A.E. Production of Phenol Oxidases and Peroxidases by Wood-Rotting Fungi. Mycologia 1989, 81, 234–240. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- RC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: https://www.R-project.org/ (accessed on 15 November 2018).
- Leisch, F.; Hornik, K.; Ripley, B.D. mda: Mixture and Flexible Discriminant Analysis; R Package Version 0.4-9; Trevor Hastie & Robert Tibshirani: Stanford, CA, USA, 2016. [Google Scholar]
Sample Availability: Not available. |



| Ion/ Compost | Control in Natura | Steam Explosion Pretreatment | Microwave:H2SO4 Pretreatment | EtOH:DMSO:AO a Pretreatment | NaOH Pretreatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count (N) | Area (μm2) | Average Size (μm2) | Count (N) | Area (μm2) | Average Size (μm2) | Count (N) | Area (μm2) | Average Size (μm2) | Count (N) | Area (μm2) | Average Size (μm2) | Count (N) | Area (μm2) | Average Size (μm2) | |
| Li+ | 353 | 53.857 | 0.150 | 88 | 0.054 | 0.027 | 63 | 0.027 | 0.027 | 320 | 30.879 | 0.097 | 16 | 4.569 | 0.286 |
| Na+ | 71 | 349.574 | 6.221 | 35 | 185.866 | 5.310 | 121 | 376.032 | 3.108 | 61 | 383.557 | 6.288 | 23 | 374.151 | 16.267 |
| K+ | 19 | 432.026 | 26.729 | 50 | 254.450 | 5.089 | 94 | 364.368 | 3.876 | 38 | 387.346 | 10.193 | 15 | 376.596 | 25.106 |
| Mg2+ | 144 | 249.479 | 2.058 | 107 | 116.690 | 1.091 | 777 | 124.457 | 0.160 | 570 | 105.242 | 0.185 | 119 | 178.072 | 1.496 |
| Ca-C3H4+ | 44 | 407.019 | 11.957 | 122 | 231.607 | 1.898 | 28 | 487.885 | 17.424 | 83 | 393.608 | 4.742 | 42 | 316.961 | 7.547 |
| F− | 197 | 278.208 | 4.107 | 209 | 167.511 | 0.801 | 778 | 168.263 | 0.216 | 299 | 244.991 | 0.819 | 46 | 386.540 | 8.403 |
| Cl− | 62 | 369.058 | 7.128 | 135 | 164.850 | 1.221 | 65 | 457.812 | 7.043 | 224 | 234.241 | 1.046 | 53 | 367.540 | 6.935 |
| DDAb | 0 | 0.000 | 0.000 | 9 | 0.403 | 0.045 | 7 | 0.215 | 0.031 | 7 | 0.403 | 0.058 | 9 | 2.365 | 0.263 |
| Pretreatment | Li+-free-OH Area (%) * |
|---|---|
| Control (in natura) | 77.92 |
| EtOH:DMSO:AO a | 78.50 |
| Steam Explosion | 84.98 |
| Microwave:H2SO4 | 85.00 |
| NaOH | 84.16 |
| Ion Salts | Laccase | Xylanase | Endoglucanase | Cellobiohydrolase | β-Glucosidase |
|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | |
| NH4F | 12.34 | 141.68 | 109.84 | 126.50 | 135.70 |
| NaH2PO4 | 37.56 | 146.81 | 99.06 | 100.10 | 141.80 |
| MgCl2∙6H2O | 41.36 | 137.39 | 61.40 | 110.90 | 144.79 |
| NH4Cl | 43.39 | 155.04 | 23.44 | 103.80 | 138.54 |
| CaCl2 | 43.07 | 147.14 | 120.15 | 119.30 | 144.23 |
| KCl | 42.72 | 148.24 | 140.31 | 109.50 | 146.57 |
| LiCl | 42.24 | 130.59 | 133.59 | 101.60 | 148.55 |
| Na2SO4 | 29.41 | 181.60 | 110.78 | 137.30 | 152.23 |
| MnCl2∙4H2O | 30.01 | 216.97 | 164.68 | 129.00 | 143.83 |
| NaCl | 38.20 | 175.46 | 134.68 | 129.70 | 150.57 |
| KH2PO4 | 33.56 | 162.18 | 124.22 | 148.30 | 143.17 |
| BaCl | 34.04 | 139.41 | 123.43 | 144.30 | 143.57 |
| Zn(NO3)2 | 40.48 | 122.86 | 119.84 | 140.10 | 137.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida Scarcella, A.S.; Somera, A.F.; da Costa Carreira Nunes, C.; Gomes, E.; Vici, A.C.; Buckeridge, M.S.; de Moraes Polizeli, M.d.L.T. Matrix Discriminant Analysis Evidenced Surface-Lithium as an Important Factor to Increase the Hydrolytic Saccharification of Sugarcane Bagasse. Molecules 2019, 24, 3614. https://doi.org/10.3390/molecules24193614
de Almeida Scarcella AS, Somera AF, da Costa Carreira Nunes C, Gomes E, Vici AC, Buckeridge MS, de Moraes Polizeli MdLT. Matrix Discriminant Analysis Evidenced Surface-Lithium as an Important Factor to Increase the Hydrolytic Saccharification of Sugarcane Bagasse. Molecules. 2019; 24(19):3614. https://doi.org/10.3390/molecules24193614
Chicago/Turabian Stylede Almeida Scarcella, Ana Sílvia, Alexandre Favarin Somera, Christiane da Costa Carreira Nunes, Eleni Gomes, Ana Claudia Vici, Marcos Silveira Buckeridge, and Maria de Lourdes Teixeira de Moraes Polizeli. 2019. "Matrix Discriminant Analysis Evidenced Surface-Lithium as an Important Factor to Increase the Hydrolytic Saccharification of Sugarcane Bagasse" Molecules 24, no. 19: 3614. https://doi.org/10.3390/molecules24193614
APA Stylede Almeida Scarcella, A. S., Somera, A. F., da Costa Carreira Nunes, C., Gomes, E., Vici, A. C., Buckeridge, M. S., & de Moraes Polizeli, M. d. L. T. (2019). Matrix Discriminant Analysis Evidenced Surface-Lithium as an Important Factor to Increase the Hydrolytic Saccharification of Sugarcane Bagasse. Molecules, 24(19), 3614. https://doi.org/10.3390/molecules24193614