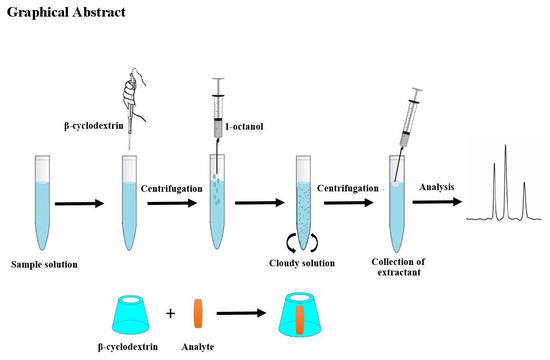
β-Cyclodextrin Assisted Liquid–Liquid Microextraction Based on Solidification of the Floating Organic Droplets Method for Determination of Neonicotinoid Residues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the β-Cyclodextrin-LLME-SFO Procedure
2.2. Analytical Performance of the Proposed Method
2.3. Application to Real Samples
2.4. Comparison of the Proposed Method with Other Methods
3. Experiments
3.1. Chemicals and Reagents
3.2. Apparatus and Chromatographic Conditions
3.3. β-Cyclodextrin-LLME-SFO Procedure
3.4. Sample Preparation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Xi, X.; Wu, X.; Lu, R.; Zhou, W.; Zhang, S.; Gao, H. Vortex-assisted magnetic β-cyclodextrin/attapulgite-linked ionic liquid dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for the fast determination of four fungicides in water samples. J. Chromatogr. A 2015, 1381, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gong, D.; Xu, B.; Chi, Q.; Zhang, X. Development of a novel, fast, sensitive method for chromium speciation in wastewater based on an organic polymer as solid phase extraction material combined with HPLC-ICP-MS. Talanta 2016, 147, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Wu, Q.; Wang, C.; Zang, X.; Wang, Z. The use of graphene-based magnetic nanoparticles as adsorbent for the extraction of triazole fungicides from environmental water. J. Sep. Sci. 2012, 35, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Margoum, C.; Guillemain, C.; Yang, X.; Coquery, M. Stir bar sorptive extraction coupled to liquid chromatography-tandem mass spectrometry for the determination of pesticides in water samples: Method validation and measurement uncertainty. Talanta 2013, 116, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Carrera, E.; León-León, V.M.; Parra, A.G.; González-Mazo, E. Simultaneous determination of pesticides, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in seawater and interstitial marine water samples, using stir bar sorptive extraction–thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2007, 1170, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, F.; Wan, Q. Ionic liquid-based ultrasound-assisted emulsification microextraction coupled with high performance liquid chromatography for the determination of four fungicides in environmental water samples. Talanta 2013, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, J.; Yang, F.; Zhang, W.; Li, W.; Liao, C.; Chen, L. Salting-out assisted liquid-liquid extraction with the aid of experimental design for determination of benzimidazole fungicides in high salinity samples by high-performance liquid chromatography. Talanta 2013, 106, 119–126. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Q.; Xie, G.; Yao, Z. Temperature-controlled ionic liquid dispersive liquid-phase microextraction combined with HPLC with ultraviolet detector for the determination of fungicides. J. Sep. Sci. 2012, 35, 2569–2574. [Google Scholar] [CrossRef]
- Yang, X.; Yang, M.; Hou, B.; Li, S.; Zhang, Y.; Lu, R.; Zhang, S. Optimization of dispersive liquid–liquid microextraction based on the solidification of floating organic droplets using an orthogonal array design and its application for the determination of fungicide concentrations in environmental water samples. J. Sep. Sci. 2014, 37, 1996–2001. [Google Scholar] [CrossRef]
- Rezaee, M.; Yamini, Y.; Faraji, M. Evolution of dispersive liquid–liquid microextraction method. J. Chromatogr. A 2010, 1217, 2342–2357. [Google Scholar] [CrossRef]
- Leong, M.I.; Huang, S.D. Dispersive liquid-liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J. Chromatogr. A 2008, 1211, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Guiñez, M.; Bazan, C.; Martinez, L.D.; Cerutti, S. Determination of nitrated and oxygenated polycyclic aromatic hydrocarbons in water samples by a liquid–liquid phase microextraction procedure based on the solidification of a floating organic drop followed by solvent assisted back-extraction and liquid chromatography–tandem mass spectrometry. Microchem. J. 2018, 139, 164–173. [Google Scholar]
- Chen, S.Y.; Chen, W.C.; Chang, S.Y. Cyclodextrin-assisted dispersive liquid-liquid microextraction for the preconcentration of carbamazepine and clobazam with subsequent sweeping micellar electrokinetic chromatography. J. Sep. Sci. 2018, 41, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Noorashikin, M.S.; Raoov, M.; Mohamad, S.; Abas, M.R. Cloud Point Extraction of Parabens Using Non-Ionic Surfactant with Cylodextrin Functionalized Ionic Liquid as a Modifier. Int. J. Mol. Sci. 2013, 14, 24531–24548. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.P. Magnetic reduced graphene oxide functionalized with β-cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phytohormones in tomatoes coupled with high performance liquid chromatography. J. Chromatogr. A 2016, 1441, 24–33. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation and Chiral Recognition Thermodynamics of 6-Amino-6-deoxy-β-cyclodextrin with Anionic, Cationic, and Neutral Chiral Guests: Counterbalance between van der Waals and Coulombic Interactions. J. Am. Chem. Soc. 2002, 124, 813–826. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J. Cyclodextrins in analytical chemistry: Host-guest type molecular recognition. Anal. Chem. 2013, 85, 8024–8030. [Google Scholar] [CrossRef]
- Chin, Y.P.; Mohamad, S.; Abas, M.R. Synthesis and Characterization of β-Cyclodextrin Functionalized Ionic Liquid Polymer as a Macroporous Material for the Removal of Phenols and As (V). Int. J. Mol. Sci. 2010, 11, 3459–3471. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.T.; Yang, X.; Dong, A.J.; Zhang, H.; Wang, J.; Liu, G.Y.; Zhai, X.C. A simple and sensitive electrochemical sensor for new neonicotinoid insecticide Paichongding in grain samples based on β-cyclodextrin-graphene modified glassy carbon electrode. Sens. Actuators B Chem. 2016, 229, 190–199. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Schmuck, R.; Lewis, G. Review of field and monitoring studies investigating the role of nitro-substituted neonicotinoid insecticides in the reported losses of honey bee colonies (Apis mellifera). Ecotoxicology 2016, 25, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Cai, W.; Cao, Y.; Wang, F.; Wang, Z.; Lu, J.; Zhang, J. An integrated strategy for rapid discovery and identification of these quential piperine metabolites in rats using ultra high-performance liquid chromatography/high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2017, 146, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Yin, X.; Wang, C.; Wang, Z. Dispersive liquid–liquid microextraction combined with sweeping micellar electrokinetic chromatography for the determination of some neonicotinoid insecticides in cucumber samples. Food Chem. 2012, 133, 544–550. [Google Scholar] [CrossRef]
- Obana, H.; Okihashi, M.; Akutsu, K.; Kitagawa, Y.; Hori, S. Determination of neonicotinoid pesticide residues in vegetables and fruits with solid phase extraction and liquid chromatography mass spectrometry. J. Agric. Food Chem. 2003, 51, 2501–2505. [Google Scholar] [CrossRef]
- Ferrone, V.; Cotellese, R.; Carlucci, M.; Di Marco, L.; Carlucci, G. Air assisted dispersive liquid-liquid microextraction with solidification of the floating organic droplets (AA-DLLME-SFO) and UHPLC-PDA method: Application to antibiotics analysis in human plasma of hospital acquired pneumonia patients. J. Pharm. Biomed. Anal. 2017, 151, 266–273. [Google Scholar] [CrossRef]
- Seccia, S.; Fidente, P.; Barbini, D.A.; Morrica, P. Multiresidue determination of nicotinoid insecticide residues in drinking water by liquid chromatography with electrospray ionization mass spectrometry. Anal. Chim. Acta 2005, 553, 21–26. [Google Scholar] [CrossRef]
- Vichapong, J.; Burakham, R.; Srijaranai, S. Vortex-assisted surfactant-enhanced-emulsification liquid-liquid microextraction with solidification of floating organic droplet combined with HPLC for the determination of neonicotinoid pesticides. Talanta 2013, 117, 221–228. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Zhang, C.; Shao, Y.; She, Y.; Jin, F.; Jin, M.; et al. Metal-organic framework UiO-66 for rapid dispersive solid phase extraction of neonicotinoid insecticides in water samples. J. Chromatogr. B 2018, 1077, 92–97. [Google Scholar] [CrossRef]
- Moyakao, K.; Santaladchaiyakit, Y.; Srijaranai, S.; Vichapong, J. Preconcentration of Trace Neonicotinoid Insecticide Residues Using Vortex-Assisted Dispersive Micro Solid-Phase Extraction with Montmorillonite as an Efficient Sorbent. Molecules 2018, 23, 883. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not from the authors. |







| Pesticide | β-Cyclodextrin-LLME-SFO | |||||||
|---|---|---|---|---|---|---|---|---|
| Linear Range (μg mL−1) | LOD (μg mL−1) | LOQ (μ g mL−1) | Intra-day (%RSD, n = 5) | Inter-day (%RSD, n = 3 × 5) | EF | |||
| tR | Peak Area | tR | Peak Area | |||||
| Thiamethoxam | 0.0015–1 | 0.0005 | 0.0015 | 0.71 | 7.15 | 0.91 | 10.99 | 10.69 |
| Clothianidin | 0.0006–1 | 0.0002 | 0.0006 | 0.68 | 3.68 | 0.86 | 6.84 | 25.93 |
| Imidacloprid | 0.0003–1 | 0.0002 | 0.0003 | 0.65 | 7.82 | 0.81 | 9.43 | 52.53 |
| Acetamiprid | 0.0003–1 | 0.0001 | 0.0003 | 0.65 | 8.38 | 0.84 | 9.50 | 44.69 |
| Thiacloprid | 0.0003–1 | 0.0001 | 0.0003 | 0.75 | 9.34 | 0.79 | 9.83 | 81.62 |
| Sample | Spiked (µg mL−1) | % Recoveries at Different Spiked Levels (% RSD) | ||||
|---|---|---|---|---|---|---|
| Thiamethoxam | Clothianidin | Imidacloprid | Acetamiprid | Thiacloprid | ||
| Surface water I | 0.000 | - | - | - | - | - |
| 0.025 | 76.65 (3.48) | 100.33 (1.15) | 75.09 (1.06) | 93.10 (1.06) | 73.57 (0.15) | |
| 0.050 | 87.18 (2.67) | 120.88 (11.6) | 97.26 (8.68) | 124.82 (2.61) | 83.42 (0.80) | |
| 0.100 | 99.69 (1.95) | 114.96 (0.97) | 91.49 (0.58) | 132.42 (1.36) | 86.88 (2.68) | |
| Surface water II | 0.000 | - | - | - | - | - |
| 0.025 | 81.86 (1.05) | 92.82 (1.07) | 100.36 (2.94) | 128.76 (0.95) | 95.18 (2.08) | |
| 0.050 | 84.86 (2.45) | 103.74 (0.63) | 96.61 (3.30) | 127.79 (4.61) | 83.40 (4.70) | |
| 0.100 | 92.03 (2.87) | 109.38 (4.97) | 99.14 (3.15) | 128.48 (4.53) | 90.73 (5.86) | |
| Surface water III | 0.000 | - | - | - | - | - |
| 0.025 | 80.92 (0.17) | 99.77 (0.17) | 94.79 (0.81) | 126.45 (3.57) | 87.99 (1.20) | |
| 0.050 | 87.80 (0.53) | 104.48 (1.86) | 98.08 (0.80) | 120.39 (0.83) | 87.41 (0.83) | |
| 0.100 | 95.19 (3.69) | 106.68 (0.73) | 99.01 (1.77) | 124.33 (4.59) | 84.13 (4.06) | |
| Method | Sample | LOD | Linearity | Recovery (%) | Ref. |
|---|---|---|---|---|---|
| VSLLME-SFO | Fruit juice and water | 0.1–0.5 (µg L−1) | 0.0005–5 (µg mL−1) | 85–105 | [28] |
| SPE | Drinking water | 0.01 µg L−1 | 0–1 (mg L−1) | 95–104 | [27] |
| DSPE | Water samples | 0.02–0.4 (ng mL−1) | 10–500 (ng mL−1) | 7–119.0 | [29] |
| VA-D-µ-SPE | Fruit juice and natural surface water | 0.005–0.065 ng mL−1 | 0.5–1000 ng mL−1 | 70–138 | [30] |
| β-cyclodextrin-LLME-SFO | Natural surface water | 0.10–0.50 (µg L−1) | 0.003–1.00 (mg L−1) | 83–132 | This study |
| Neonicotinoid Insecticide | Water Solubility (mg L−1) at 20 °C | Log KOW | Structure |
|---|---|---|---|
| Thiamethoxam | 4100 | −0.13 |  |
| Imidacloprid | 610 | 0.57 |  |
| Clothianidin | 340 | 0.91 |  |
| Acetamiprid | 2950 | 0.80 |  |
| Thiacloprid | 184 | 1.26 |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vichapong, J.; Moyakao, K.; Kachangoon, R.; Burakham, R.; Santaladchaiyakit, Y.; Srijaranai, S. β-Cyclodextrin Assisted Liquid–Liquid Microextraction Based on Solidification of the Floating Organic Droplets Method for Determination of Neonicotinoid Residues. Molecules 2019, 24, 3954. https://doi.org/10.3390/molecules24213954
Vichapong J, Moyakao K, Kachangoon R, Burakham R, Santaladchaiyakit Y, Srijaranai S. β-Cyclodextrin Assisted Liquid–Liquid Microextraction Based on Solidification of the Floating Organic Droplets Method for Determination of Neonicotinoid Residues. Molecules. 2019; 24(21):3954. https://doi.org/10.3390/molecules24213954
Chicago/Turabian StyleVichapong, Jitlada, Khwankaew Moyakao, Rawikan Kachangoon, Rodjana Burakham, Yanawath Santaladchaiyakit, and Supalax Srijaranai. 2019. "β-Cyclodextrin Assisted Liquid–Liquid Microextraction Based on Solidification of the Floating Organic Droplets Method for Determination of Neonicotinoid Residues" Molecules 24, no. 21: 3954. https://doi.org/10.3390/molecules24213954
APA StyleVichapong, J., Moyakao, K., Kachangoon, R., Burakham, R., Santaladchaiyakit, Y., & Srijaranai, S. (2019). β-Cyclodextrin Assisted Liquid–Liquid Microextraction Based on Solidification of the Floating Organic Droplets Method for Determination of Neonicotinoid Residues. Molecules, 24(21), 3954. https://doi.org/10.3390/molecules24213954