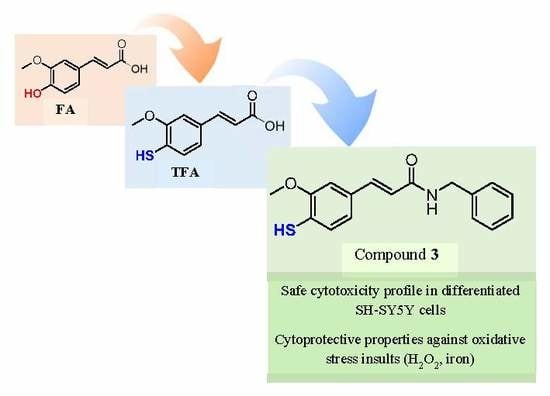
Insights into the Discovery of Novel Neuroprotective Agents: A Comparative Study between Sulfanylcinnamic Acid Derivatives and Related Phenolic Analogues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Evaluation of Antioxidant Activity
2.3. Electrochemical Studies
2.4. Cellular Studies
2.4.1. Evaluation of Cytotoxicity Profile
2.4.2. Protection against H2O2- and Ferric Iron-Induced Oxidative Damage
2.5. Estimation of Drug-Like Properties
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis
I. Synthesis of 6-Hydroxy-5-methoxy-[1,1′-biphenyl]-3-carbaldehyde (12)
II. Thiocarbamoylation of Phenols
III. Microwave-Assisted Newman-Kwart Rearrangement
IV. Knoevenagel-Doebner Condensation
V. PyBOP-Mediated Cinnamic Acid Amidation
VI. Alkaline Hydrolysis
3.2. Radical Scavenging Activity
3.2.1. Spectrophotometric Methods
I. DPPH• Radical Assay
II. ABTS•+ Radical Cation Assay
III. GO• Radical Assay
3.2.2. Fluorometric Methods (ORAC-FL Assay)
3.3. Electrochemical Measurements
3.4. In Vitro Toxicology
3.4.1. SH-SY5Y Cell Culture
3.4.2. Cytotoxicity
3.4.3. Protection against Oxidative Stress Inducers
I. Cytoprotective Properties against H2O2-Induced Damage
II. Cytoprotective Properties against FeNTA-Induced Damage
3.4.4. Statistical Analysis
3.5. Estimation of Drug-Like Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases: A mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Acrolein is increased in Alzheimer‘s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Lovell, M.A. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 1998, 19, 33–36. [Google Scholar] [CrossRef]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Good, P.F.; Werner, P.; Hsu, A.; Olanow, C.W.; Perl, D.P. Evidence of neuronal oxidative damage in Alzheimer’s disease. Am. J. Pathol. 1996, 149, 21–28. [Google Scholar]
- Good, P.F.; Hsu, A.; Werner, P.; Perl, D.P.; Olanow, C.W. Protein nitration in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1998, 57, 338–342. [Google Scholar] [CrossRef]
- Kim, T.S.; Pae, C.U.; Yoon, S.J.; Jang, W.Y.; Lee, N.J.; Kim, J.J.; Lee, S.J.; Lee, C.; Paik, I.H.; Lee, C.U. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 344–348. [Google Scholar] [CrossRef]
- Marcus, D.L.; Thomas, C.; Rodriguez, C.; Simberkoff, K.; Tsai, J.S.; Strafaci, J.A.; Freedman, M.L. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp. Neurol. 1998, 150, 40–44. [Google Scholar] [CrossRef]
- Venkateshappa, C.; Harish, G.; Mahadevan, A.; Srinivas Bharath, M.M.; Shankar, S.K. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: Implications for neurodegeneration in Alzheimer’s disease. Neurochem. Res. 2012, 37, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Venkateshappa, C.; Harish, G.; Mythri, R.B.; Mahadevan, A.; Bharath, M.M.; Shankar, S.K. Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: Implications for Parkinson’s disease. Neurochem. Res. 2012, 37, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Pocernich, C.B.; Lange, M.L.; Sultana, R.; Butterfield, D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Majekova, M.; Medina, M.; Valoti, M. Key Targets for Multi-Target Ligands Designed to Combat Neurodegeneration. Front. Neurosci. 2016, 10, 1–24. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Haque, M.E. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Devel. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef]
- Nagarajan, S.; Chellappan, D.R.; Chinnaswamy, P.; Thulasingam, S. Ferulic acid pretreatment mitigates MPTP-induced motor impairment and histopathological alterations in C57BL/6 mice. Pharm. Biol. 2015, 53, 1591–1601. [Google Scholar] [CrossRef]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Vashisth, P.; Kumar, N.; Sharma, M.; Pruthi, V. Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol. Rep. (Amst) 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Yakub, G.; Ignatova, M.; Manolova, N.; Rashkov, I.; Toshkova, R.; Georgieva, A.; Markova, N. Chitosan/ferulic acid-coated poly(ε-caprolactone) electrospun materials with antioxidant, antibacterial and antitumor properties. Int. J. Biol. Macromol. 2018, 107, 689–702. [Google Scholar] [CrossRef]
- Silva, T.; Bravo, J.; Summavielle, T.; Remião, F.; Pérez, C.; Gil, C.; Martínez, A.; Borges, F. Biology-oriented development of novel lipophilic antioxidants with neuroprotective activity. RSC Adv. 2015, 5, 15800–15811. [Google Scholar] [CrossRef]
- Gaspar, A.; Garrido, E.M.; Esteves, M.; Quezada, E.; Milhazes, N.; Garrido, J.; Borges, F. New insights into the antioxidant activity of hydroxycinnamic acids: Synthesis and physicochemical characterization of novel halogenated derivatives. Eur. J. Med. Chem. 2009, 44, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, D.; Fernandes, C.; Silva, T.; Garrido, J.; Remião, F.; Oliveira, P.J.; Borges, F. Bioisosteric OH- to SH- replacement changes the antioxidant profile of ferulic acid. Org. Biomol. Chem. 2019, 17, 9646–9654. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Liu, X.; Fang, Q.; Wang, Z.; Fu, L.; Liu, Z.; Wang, Y.; Zhao, Y.; Li, X.; et al. Discovery of a New Inhibitor of Myeloid Differentiation 2 from Cinnamamide Derivatives with Anti-Inflammatory Activity in Sepsis and Acute Lung Injury. J. Med. Chem. 2016, 59, 2436–2451. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical Methods for Total Antioxidant Capacity and its Main Contributors Determination: A review. Open Chem. 2015, 13, 824–856. [Google Scholar] [CrossRef]
- Luc, R.; Vergely, C. Forgotten radicals in biology. Int. J. Biomed. Sci. 2008, 4, 255–259. [Google Scholar]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. Biomed. Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fernandes, C.; Pinto, M.; Martins, C.; Gomes, M.J.; Sarmento, B.; Oliveira, P.J.; Remião, F.; Borges, F. Development of a PEGylated-Based Platform for Efficient Delivery of Dietary Antioxidants Across the Blood-Brain Barrier. Bioconjug. Chem. 2018, 29, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Belyanskaya, L.; Manser, P.; Spohn, P.; Bruinink, A.; Wick, P. The reliability and limits of the MTT reduction assay for carbon nanotubes-cell interaction. Carbon 2007, 45, 2643–2648. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Nunez, M.T.; Gallardo, V.; Munoz, P.; Tapia, V.; Esparza, A.; Salazar, J.; Speisky, H. Progressive iron accumulation induces a biphasic change in the glutathione content of neuroblastoma cells. Free Radic. Biol. Med. 2004, 37, 953–960. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Hitchcock, S.A.; Pennington, L.D. Structure-brain exposure relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal Chemical Properties of Successful Central Nervous System Drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Nowakowska, Z. The conversion of stilbenols to stilbenethiols via N,N-dimethylthiocarbamates. Phosphorus Sulfur 2006, 181, 707–715. [Google Scholar] [CrossRef]
- Teixeira, J.; Silva, T.; Benfeito, S.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Exploring nature profits: Development of novel and potent lipophilic antioxidants based on galloyl-cinnamic hybrids. Eur. J. Med. Chem. 2013, 62, 289–296. [Google Scholar] [CrossRef]
- Gaspar, A.; Silva, T.; Yanez, M.; Vina, D.; Orallo, F.; Ortuso, F.; Uriarte, E.; Alcaro, S.; Borges, F. Chromone, a privileged scaffold for the development of monoamine oxidase inhibitors. J. Med. Chem. 2011, 54, 5165–5173. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative properties of vanillic acid esters in multiple antioxidant assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.Y.; Liu, Z.Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, Z.G.; Liu, Z.Q. Synthesis and antioxidant capacities of hydroxyl derivatives of cinnamoylphenethylamine in protecting DNA and scavenging radicals. Free Radic. Res. 2011, 45, 445–453. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Mura, F.; Silva, T.; Castro, C.; Borges, F.; Zuniga, M.C.; Morales, J.; Olea-Azar, C. New insights into the antioxidant activity of hydroxycinnamic and hydroxybenzoic systems: Spectroscopic, electrochemistry, and cellular studies. Free Radic. Res. 2014, 48, 1473–1484. [Google Scholar] [CrossRef]
- Fernandes, C.; Martins, C.; Fonseca, A.; Nunes, R.; Matos, M.J.; Silva, R.; Garrido, J.; Sarmento, B.; Remiao, F.; Otero-Espinar, F.J.; et al. PEGylated PLGA Nanoparticles As a Smart Carrier to Increase the Cellular Uptake of a Coumarin-Based Monoamine Oxidase B Inhibitor. ACS Appl. Mater. Interfaces 2018, 10, 39557–39569. [Google Scholar] [CrossRef]





| Compound |  | IC50 (µM) | ORAC-FL Index | Ep (mV) | ||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | DPPH• | ABTS•+ | GO• | |||
| 2 (TFA) |  |  |  | 47.8 ± 0.9a | 19.9 ± 1.0a | 4.25 ± 0.10a | 0.34 ± 0.04a | 271 |
| 3 |  |  |  | 49.8 ± 0.7 | 20.6 ± 0.7 | 4.50 ± 0.08 | 0.12 ± 0.02 | 198 |
| 4 |  |  |  | 47.3 ± 0.7 | 19.9 ± 0.3 | 4.41 ± 0.15 | 0.15 ± 0.01 | 186 |
| 5 |  |  |  | 42.7 ± 2.1 | 22.9 ± 0.6 | 6.70 ± 0.07 | 0.30 ± 0.03 | 218 |
| 6 |  |  |  | 50.7 ± 1.6 | 27.0 ± 1.8 | 6.66 ± 0.13 | 0.34 ± 0.01 | 245 |
| 1(FA) |  |  |  | 64.6 ± 1.9a | 16.8 ± 0.4a | 37.5 ± 2.5a | 3.2 ± 0.1a | 344 b |
| 7 |  |  |  | 61.7 ± 0.8 | 25.6 ± 1.8 | 76.4 ± 3.6 | 4.0 ± 0.4 | 327 |
| 8 |  |  |  | 61.2 ± 0.7 | 26.0 ± 1.7 | 70.7 ± 2.5 | 3.7 ± 0.3 | 338 |
| 9 |  |  |  | 43.9 ± 1.6 | 16.6 ± 0.3 | 3.90 ± 0.1 | 3.2 ± 0.3 | 291 |
| 10 |  |  |  | 69.7 ± 3.8 | 19.8 ± 0.8 | 31.2 ± 3.9 | 2.7 ± 0.1 | 351 |
| Trolox | ---- | ---- | ---- | 24.6 ± 0.9 | 18.2 ± 0.5 | 2.98 ± 0.15 | 1.00 ± 0.02 | 110 |
| Compound | MW a | cLogPb | TPSA a | HBA a | HBD a | RB | LogBB |
|---|---|---|---|---|---|---|---|
| 2 | 210.25 | 1.97 | 46.53 | 4 | 2 | 3 | −0.9373 |
| 3 | 299.39 | 3.25 | 38.33 | 4 | 2 | 6 | 0.02948 |
| 10 | 270.28 | 2.76 | 66.76 | 4 | 2 | 4 | -1.511 |
| CNS+ drugs | < 500 c | 2–5 c | < 90 c | < 7 d | < 3 d | < 8 d | <−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavarria, D.; Fernandes, C.; Aguiar, B.; Silva, T.; Garrido, J.; Remião, F.; Oliveira, P.J.; Uriarte, E.; Borges, F. Insights into the Discovery of Novel Neuroprotective Agents: A Comparative Study between Sulfanylcinnamic Acid Derivatives and Related Phenolic Analogues. Molecules 2019, 24, 4405. https://doi.org/10.3390/molecules24234405
Chavarria D, Fernandes C, Aguiar B, Silva T, Garrido J, Remião F, Oliveira PJ, Uriarte E, Borges F. Insights into the Discovery of Novel Neuroprotective Agents: A Comparative Study between Sulfanylcinnamic Acid Derivatives and Related Phenolic Analogues. Molecules. 2019; 24(23):4405. https://doi.org/10.3390/molecules24234405
Chicago/Turabian StyleChavarria, Daniel, Carlos Fernandes, Brandon Aguiar, Tiago Silva, Jorge Garrido, Fernando Remião, Paulo J. Oliveira, Eugenio Uriarte, and Fernanda Borges. 2019. "Insights into the Discovery of Novel Neuroprotective Agents: A Comparative Study between Sulfanylcinnamic Acid Derivatives and Related Phenolic Analogues" Molecules 24, no. 23: 4405. https://doi.org/10.3390/molecules24234405
APA StyleChavarria, D., Fernandes, C., Aguiar, B., Silva, T., Garrido, J., Remião, F., Oliveira, P. J., Uriarte, E., & Borges, F. (2019). Insights into the Discovery of Novel Neuroprotective Agents: A Comparative Study between Sulfanylcinnamic Acid Derivatives and Related Phenolic Analogues. Molecules, 24(23), 4405. https://doi.org/10.3390/molecules24234405