Spectrophotometric and Smartphone-Assisted Determination of Phenolic Compounds Using Crude Eggplant Extract
Abstract
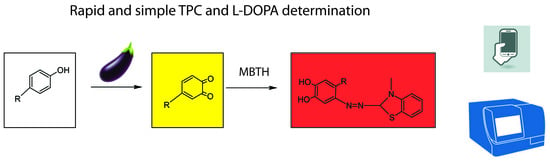
:1. Introduction
2. Results and Discussion
2.1. Eggplant Extract Polyphenol Oxidase Activity and Substrate Specificity
2.2. Eggplant Extract Polyphenol Oxidase Interaction with Catechol, Caffeic Acid, Chlorogenic Acid, and l-DOPA
2.3. Analytical Application
2.3.1. Cuvette and Microplate Procedures with Spectrophotometer
2.3.2. Smartphone-Assisted Microplate Procedure
3. Materials and Methods
3.1. Reagents and equipment
3.2. Crude Eggplant Extract Preparation
3.3. General Procedure for Crude Plant Extract and Phenolic Compounds Interaction Study
3.4. Samples Preparation
3.5. Procedures of Phenolic Compounds Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| CA | Caffeic acid |
| Cat | Catechol |
| ChA | Chlorogenic acid |
| l-DOPA | l-Dihydroxyphenylalanine |
| EE | Crude eggplant extract |
| HSV | Hue saturation value |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MBTH | 3-methyl-2-benzothiazolinone hydrazone |
| PPO | Polyphenol oxidase |
| TPC | Total polyphenol content |
References
- David, I.G.; Bizgan, A.C.; Popa, D.E.; Buleandra, M.; Moldovan, Z.; Badea, I.A.; Tekiner, T.A.; Basaga, H.; Ciucu, A.A. Rapid determination of total polyphenolic content in tea samples based on caffeic acid voltammetric behaviour on a disposable graphite electrode. Food Chem. 2015, 173, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Granero, A.M.; Fernández, H.; Agostini, E.; Zón, M.A. An amperometric biosensor based on peroxidases from Brassica napus for the determination of the total polyphenolic content in wine and tea samples. Talanta 2010, 83, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, S.; Santhosh, A.S.; Swamy, N.K.; Suresh, G.S.; Melo, J.S.; Nithin, K.S. Electrochemical detection of L-dopa using crude Polyphenol oxidase enzyme immobilized on electrochemically reduced RGO-Ag nanocomposite modified graphite electrode. Mater. Sci. Eng. B 2018, 232–235, 15–21. [Google Scholar] [CrossRef]
- Morosanova, M.A.; Fedorov, A.S.; Morosanova, E.I. Crude Plant Extracts Mediated Polyphenol Oxidation Reactions in the Presence of 3-Methyl-2-Benzothiazolinone Hydrazone for the Determination of Total Polyphenol Content in Beverages. Curr. Anal. Chem. 2019, 15, 11–20. [Google Scholar] [CrossRef]
- Di Fusco, M.; Tortolini, C.; Deriu, D.; Mazzei, F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 2010, 81, 235–240. [Google Scholar] [CrossRef] [PubMed]
- ElKaoutit, M.; Naranjo-Rodriguez, I.; Temsamani, K.R.; Hernández-Artiga, M.P.; Bellido-Milla, D.; Hidalgo-Hidalgo de Cisneros, J.L. A comparison of three amperometric phenoloxidase–Sonogel–Carbon based biosensors for determination of polyphenols in beers. Food Chem. 2008, 110, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Montereali, M.R.; Della Seta, L.; Vastarella, W.; Pilloton, R. A disposable Laccase–Tyrosinase based biosensor for amperometric detection of phenolic compounds in must and wine. J. Mol. Catal. B Enzym. 2010, 64, 189–194. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O. Consideration on equivalent chemicals in total phenolic assay of chlorogenic acid-rich plums. Food Res. Int. 2004, 37, 337–342. [Google Scholar] [CrossRef]
- Draghi, P.F.; Fernandes, J.C.B. Label-free potentiometric biosensor based on solid-contact for determination of total phenols in honey and propolis. Talanta 2017, 164, 413–417. [Google Scholar] [CrossRef]
- Chawla, S.; Rawal, R.; Shabnam; Kuhad, R.C.; Pundir, C.S. An amperometric polyphenol biosensor based on laccase immobilized on epoxy resin membrane. Anal. Methods 2011, 3, 709–714. [Google Scholar] [CrossRef]
- Gul, I.; Ahmad, M.S.; Naqvi, S.M.S.; Hussain, A.; Wali, R.; Farooqi, A.A.; Ahmed, I. Polyphenol oxidase (PPO) based biosensors for detection of phenolic compounds: A Review. J. Appl. Biol. Biotechnol. 2017, 5, 72–85. [Google Scholar] [CrossRef]
- Perez-Gilabert, M.; Carmona, F.G. Characterization of Catecholase and Cresolase Activities of Eggplant Polyphenol Oxidase. J. Agric. Food Chem. 2000, 48, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.C.; Wisniewski, C.; Luccas, P.O.; de Magalhães, C.S. Enzyme from Banana (Musa sp.) Extraction Procedures for Sensitive Adrenaline Biosensor Construction. Am. J. Analyt. Chem. 2013, 4, 293–300. [Google Scholar] [CrossRef]
- Antunes, R.S.; Garcia, L.F.; Somerset, V.S.; de Souza Gil, E.; Lopes, F.M. The Use of a Polyphenoloxidase Biosensor Obtained from the Fruit of Jurubeba (Solanum paniculatum L.) in the Determination of Paracetamol and Other Phenolic Drugs. Biosensors 2018, 8, 36. [Google Scholar] [CrossRef]
- Da Cruz Vieira, I.; Fatibello-Filho, O. Amperometric Biosensor for the Determination of Phenols Using a Crude Extract of Sweet Potato (Ipomoea Batatas (L.) Lam.). Anal. Lett. 1997, 30, 895–907. [Google Scholar] [CrossRef]
- Sulzbacher Caruso, C.; da Cruz Vieira, I.; Fatibello-Filho, O. Determination of Epinephrine and Dopamine in Pharmaceutical Formulations Using a Biosensor Based on Carbon Paste Modified with Crude Extract of Cara Root (Dioscorea bulbifera). Anal. Lett. 1999, 32, 39–50. [Google Scholar] [CrossRef]
- Todaro, A.; Cavallaro, R.; Argento, S.; Branca, F.; Spagna, G. Study and Characterization of Polyphenol Oxidase from Eggplant (Solanum melongena L.). J. Agric. Food Chem. 2011, 59, 11244–11248. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Purification and characterisation of polyphenol oxidase (PPO) from eggplant (Solanum melongena). Food Chem. 2012, 134, 1855–1861. [Google Scholar] [CrossRef]
- Garcia, L.F.; Benjamin, S.R.; Antunes, R.S.; Lopes, F.M.; Somerset, V.S.; de Souza Gil, E. Solanum melongena polyphenol oxidase biosensor for the electrochemical analysis of paracetamol. Prep. Biochem. Biotechnol. 2016, 46, 850–855. [Google Scholar] [CrossRef]
- Navaratne, A.; Lin, M.S.; Rechnitz, G.A. Eggplant-based bioamperometric sensor for the detection of catechol. Anal. Chim. Acta 1990, 237, 107–113. [Google Scholar] [CrossRef]
- Kuntzleman, T.S.; Jacobson, E.C. Teaching Beer’s law and absorption spectrophotometry with a smart phone: a substantially simplified protocol. J. Chem. Educ. 2016, 93, 1249–1252. [Google Scholar] [CrossRef]
- Haifa, B.A.; Bacarea, V.; Iacob, O.; Calinici, T.; Schiopu, A. Comparison between Digital Image Processing and Spectrophotometric Measurements Methods. Application in Electrophoresis Interpretation. Appl. Med. Inform. 2011, 28, 29–36. [Google Scholar]
- Nirmal, N.P.; Benjakul, S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009, 116, 323–331. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Matyushina, T.A.; Morosanova, E.I.; Zolotov, Y.A. Microsequential injection analysis: Determination of rutin and quercetin in food supplements and pharmaceutical products. J. Anal. Chem. 2010, 65, 308–315. [Google Scholar] [CrossRef]
- Pifferi, P.G.; Baldassari, L. A spectrophotometric method for the determination of the catecholase activity of tyrosinase by Besthorn’s hydrazone. Anal. Biochem. 1973, 52, 325–335. [Google Scholar] [CrossRef]
- Setti, L.; Scali, S.; Angeli, I.D.; Pifferi, P.G. Horseradish peroxidase-catalyzed oxidative coupling of 3-methyl 2-benzothiazolinone hydrazone and methoxyphenols. Enzyme Microb. Technol. 1998, 22, 656–661. [Google Scholar] [CrossRef]
- Abdullah, J.; Ahmad, M.; Heng, L.Y.; Karuppiah, N.; Sidek, H. Stacked films immobilization of MBTH in nafion/sol-gel silicate and horseradish peroxidase in chitosan for the determination of phenolic compounds. Anal. Bioanal. Chem. 2006, 386, 1285–1292. [Google Scholar] [CrossRef]
- Abdullah, J.; Ahmad, M.; Heng, L.Y.; Karuppiah, N.; Sidek, H. An Optical Biosensor based on Immobilization of Laccase and MBTH in Stacked Films for the Detection of Catechol. Sensors 2007, 7, 2238–2250. [Google Scholar] [CrossRef]
- Setti, L.; Giuliani, S.; Spinozzi, G.; Pifferi, P.G. Laccase catalyzed-oxidative coupling of 3-methyl 2-benzothiazolinone hydrazone and methoxyphenols. Enzyme Microb. Technol. 1999, 25, 285–289. [Google Scholar] [CrossRef]
- Abdullah, J.; Ahmad, M.; Heng, L.Y.; Karuppiah, N.; Sidek, H. Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sens. Actuat. B Chem. 2006, 114, 604–609. [Google Scholar] [CrossRef]
- Fiorentino, D.; Gallone, A.; Fiocco, D.; Palazzo, G.; Mallardi, A. Mushroom tyrosinase in polyelectrolyte multilayers as an optical biosensor for o-diphenols. Biosens. Bioelectron. 2010, 25, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, A.; Tian, M.; Liu, C.; Wang, Y. Effect of ethanol treatment on physiological and quality attributes of fresh-cut eggplant. J. Sci. Food Agric. 2010, 90, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.; Kumar, V. Automated doctors: cell phones as diagnostic tools. Clin. Chem. 2012, 58, 1607–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Yan-Lok Chan, R.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-Based Hand-Held Microplate Reader for Point-of-Care Testing of Enzyme-Linked Immunosorbent Assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, D.; Jónás, L.; Los, M.; Montangero, M.; Szabó, M.G. How Deep Is Your Blue?—Coloured Chemistry with Smartphones. Science on Stage Deutschland 2014, 4, 20–24. [Google Scholar]
- Cantrell, K.; Erenas, M.M.; de Orbe-Payá, I.; Capitán-Vallvey, L.F. Use of the Hue Parameter of the Hue, Saturation, Value Color Space As a Quantitative Analytical Parameter for Bitonal Optical Sensors. Anal. Chem. 2010, 82, 531–542. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Amini, M.K.; Kia, R.; Tangestaninejad, S. Salicylate-Selective Electrodes Based on Al(III) and Sn(IV) Salophens. Anal. Chem. 2000, 72, 956–962. [Google Scholar] [CrossRef]
- Uppuluri, P.; Dinakaran, H.; Thomas, D.P.; Chaturvedi, A.K.; Lopez-Ribot, J.L. Characteristics of Candida albicans Biofilms Grown in a Synthetic Urine Medium. J. Clin. Microbiol. 2009, 47, 4078–4083. [Google Scholar] [CrossRef] [Green Version]






| Substrate | Eggplant Extract | Eggplant Extract + MBTH | ||
|---|---|---|---|---|
| Vmax, min−1 | Km, M | Vmax, min−1 | Km, M | |
| Catechol | 0.167 | 1.3∙10−3 | 0.561 | 3.9∙10−4 |
| Caffeic acid | 0.315 | 1.6∙10−3 | 0.317 | 4.8∙10−4 |
| Chlorogenic acid | 0.496 | 1.4∙10−3 | 0.577 | 6.4∙10−4 |
| l-DOPA | 0.115 | 8.6·10−4 | 0.131 | 5.1∙10−4 |
| Substrate | Spectrophotometric Determination | Smartphone Determination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggplant Extract (Cuvette) | Eggplant Extract + MBTH (Cuvette) | Eggplant Extract + MBTH (Microplate) | Eggplant Extract + MBTH (Microplate) | |||||||||
| Analytical Range, M∙105 | LOD, M∙105 | LOQ, M∙105 | Analytical Range, M∙105 | LOD, M∙105 | LOQ, M∙105 | Analytical Range, M∙105 | LOD, M∙105 | LOQ, M∙105 | Analytical Range, M∙105 | LOD, M∙105 | LOQ, M∙105 | |
| Catechol | 14.3–125.0 | 4.8 | 14.3 | 1.5–125.0 | 0.5 | 1.5 | 3.6–50.0 | 1.2 | 3.6 | 7.2–50.0 | 2.4 | 7.2 |
| Caffeic acid | 12.0–100.0 | 4.0 | 12.0 | 1.9–50.0 | 0.6 | 1.9 | 3.9–50.0 | 1.3 | 3.9 | 7.8–50.0 | 2.6 | 7.8 |
| Chlorogenic acid | 9.5–50.0 | 3.2 | 9.5 | 1.4–50.0 | 0.5 | 1.4 | 3.9–25.0 | 1.3 | 3.9 | 6.3–25.0 | 2.1 | 6.3 |
| l-DOPA | 22.8–125.0 | 7.6 | 22.8 | 9.0–125.0 | 3.0 | 9.0 | 24.0–100.0 | 8.0 | 24.0 | 36.3–100.0 | 12.1 | 36.3 |
| Interfering Substance | Threshold (Concentration Causing 10% Error) |
|---|---|
| Ascorbic acid | 2.0·10−4 M |
| Citric acid | 1.8·10−3 M |
| Tartaric acid | 1.8·10−3 M |
| Sulfite | 1.9·10−4 M |
| Ethanol | 7.0 %vol. |
| Analyte | Sample | Found With Cuvette Procedure (RSD, %) | Found With Microplate Procedure (RSD, %) | Found With Smartphone Procedure (RSD, %) | Reference Value (RSD, %) |
|---|---|---|---|---|---|
| TPC in CA equivalents | Green tea | 31.0 ± 5.3 mg/g (10.1) | 30.8 ± 7.3 mg/g (14.0) | 38.3 ± 11.3 mg/g (17.6) | 31.9 ± 1.3 mg/g (2.4)a |
| Black tea | 25.5 ± 2.7 mg/g (6.2) | - | - | 26.3 ± 1.8 mg/g (4.0)a | |
| TPC in ChA equivalents | Green coffee extract tablets | 136 ± 10 mg/tablet (5.4) | 140 ± 19 mg/tablet (9.9) | 120 ± 20 mg/tablet (11.8) | 130 mg/tabletb |
| Caffeic acid | Synthetic urine sample | - | (1.1 ± 0.1)·10−4 M (5.4) | (1.2 ± 0.2)·10−4 M (8.7) | 1.0·10−4 Mc |
| l-DOPA | Synthetic serum sample 1 | (2.4 ± 0.5)·10−4 M (12.9) | - | - | 2.5·10−4 Mc |
| Synthetic serum sample 2 | (5.2 ± 0.3)·10−4 M (2.9) | (5.0 ± 0.7)·10−4 M (8.6) | (4.9 ± 1.1)·10−4 M (12.7) | 5.0·10−4 Mc | |
| Synthetic urine sample 1 | (5.4 ± 0.4)·10−4 M (4.7) | (4.9 ± 0.8)·10−4 M (10.5) | (5.4 ± 1.2)·10−4 M (13.3) | 5.0·10−4 Mc | |
| Synthetic urine sample 2 | (9.7 ± 0.8)·10−4 M (5.0) | - | - | 1.0·10−3 Mc |
| Enzyme | Time of Analysis, min | Analyte | LOD, µM | Reference |
|---|---|---|---|---|
| Laccase | 10 | Catechol | 330 | [29] |
| Tyrosinase | 15 | 4-Chlorophenol | 0.9 | [31] |
| Phenol | 1.0 | |||
| m-Cresol | 1.0 | |||
| p-Cresol | 3.0 | |||
| Tyrosinase | 2 | l-DOPA | 23 | [32] |
| Horseradish peroxidase | 5 | Guaiacol | 10 | [28] |
| Resorcinol | 5 | |||
| o-Cresol | 12 | |||
| Banana crude extract | 1 | Catechol | 4.6 | [4] |
| Gallic acid | 29 | |||
| Green bean crude extract | 1 | Caffeic acid | 5.0 | |
| Ferulic acid | 3.9 | |||
| Quercetin | 1.0 | |||
| Eggplant crude extract | 4 | Catechol | 5.1 | Present work(cuvette variant) |
| Caffeic acid | 5.3 | |||
| Chlorogenic acid | 4.6 | |||
| l-DOPA | 30.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morosanova, M.A.; Bashkatova, A.S.; Morosanova, E.I. Spectrophotometric and Smartphone-Assisted Determination of Phenolic Compounds Using Crude Eggplant Extract. Molecules 2019, 24, 4407. https://doi.org/10.3390/molecules24234407
Morosanova MA, Bashkatova AS, Morosanova EI. Spectrophotometric and Smartphone-Assisted Determination of Phenolic Compounds Using Crude Eggplant Extract. Molecules. 2019; 24(23):4407. https://doi.org/10.3390/molecules24234407
Chicago/Turabian StyleMorosanova, M.A., A.S. Bashkatova, and E.I. Morosanova. 2019. "Spectrophotometric and Smartphone-Assisted Determination of Phenolic Compounds Using Crude Eggplant Extract" Molecules 24, no. 23: 4407. https://doi.org/10.3390/molecules24234407
APA StyleMorosanova, M. A., Bashkatova, A. S., & Morosanova, E. I. (2019). Spectrophotometric and Smartphone-Assisted Determination of Phenolic Compounds Using Crude Eggplant Extract. Molecules, 24(23), 4407. https://doi.org/10.3390/molecules24234407