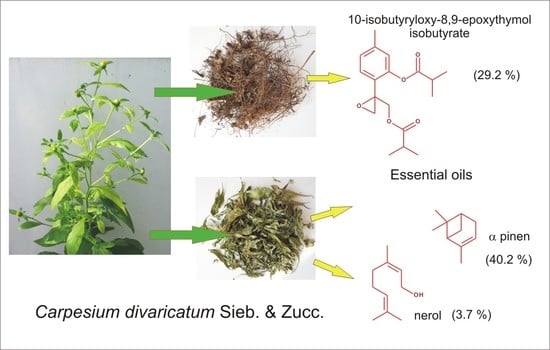
Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Isolation of Essential Oil
4.4. Isolation and NMR Analysis of Volatile Components
4.5. Identification of Essential Oil Constituents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stojanović-Radić, Z.; Čomić, L.j.; Radulović, N.; Blagojević, P.; Denić, M.; Miltojević, A.; Rajkowić, J.; Mihajilov-Krstev, T. Antistaphylococcal activity of Inula helenium L. root essential oil: Eudesmane sesquiterpene lactones induce cell membrane damage. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Awadh Ali, N.A.; Crouch, R.A.; Al-Fatimi, M.A.; Arnold, N.; Teichert, A.; Setzer, W.N.; Wessjohann, L. Chemical composition, antimicrobial, antiradical and anticholinesterase activity of the essential oil of Pulicaria stephanocarpa from Soqotra. Nat. Prod. Commun. 2012, 7, 113–116. [Google Scholar]
- Pang, Y.; Wang, D.; Hu, X.; Wang, H.; Fu, W.; Fan, Z.; Chen, X.; Yu, F. Effect of volatile oil from Blumea balsamifera (L.) DC. leaves on wound healing in mice. J. Tradit. Chin. Med. 2014, 34, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Kameoka, H.; Sagara, K.; Miyazawa, M. Components of essential oils of Kakushitsu (Daucus carota L. and Carpesium abrotanoides L.). Nippon Nōgeikagaku Kaishi 1989, 63, 185–188. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, L.; Lin, L.; Zhang, R.; Du, Y.; Chen, H.; Huang, M.; Guo, K.; Yang, X. Essential oil from Carpesium abrotanoides L. induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018, 38, 1045–1055. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Wang, G.-W.; Tian, X.-H.; Yang, Y.-X.; Liu, Q.-X.; Chen, L.-P.; Li, H.-L.; Zhang, W.-D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Kim, H.; Song, M.-J. Ethnobotanical analysis for traditional knowledge of wild edible plants in North Jeolla Province (Korea). Genet. Resour. Crop Evol. 2013, 60, 1571–1585. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Y.; Ranjitkar, S.; Huai, H.; Wang, Y. Traditional knowledge and its transmission of wild edibles used by the Naxi in Baidi Village, northwest Yunnan province. J. Ethnobiol. Ethnomed. 2016, 12, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Chen, J.-H.; Si, J.-G.; Ding, G.; Zhang, Q.-B.; Zhang, H.-W.; Jia, H.-M.; Zou, Z.-M. Isolation, structure elucidation, and absolute configuration of germacrane isomers from Carpesium divaricatum. Sci. Rep. 2018, 8, 12418. [Google Scholar] [CrossRef] [PubMed]
- Kłeczek, N.; Michalak, B.; Malarz, J.; Kiss, A.K.; Stojakowska, A. Carpesium divaricatum Sieb. & Zucc. revisited: New constituents from aerial parts of the plant and their possible contribution to the biological activity of the plant. Molecules 2019, 24, 1614. [Google Scholar] [CrossRef] [Green Version]
- Anderberg, A.A.; Eldenäs, P.; Bayer, R.J.; Englund, M. Evolutionary relationships in the Asteraceae tribe Inuleae (incl. Plucheeae) evidenced by DNA sequences of ndhF; with notes on the systematic positions of some aberrant genera. Org. Divers. Evol. 2005, 5, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Larruscain, D.; Santos-Vicente, M.; Anderberg, A.A.; Rico, E.; Martínez-Ortega, M.M. Phylogeny of the Inula group (Asteraceae: Inuleae): Evidence from nuclear and plastid genomes and a recircumscription of Pentanema. Taxon 2018, 67, 149–164. [Google Scholar] [CrossRef]
- Orhan, I.; Küpeli, E.; Aslan, M.; Kartal, M.; Yesilada, E. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L. J. Ethnopharm. 2006, 105, 235–240. [Google Scholar] [CrossRef]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily inhalation of α-pinene in mice: Effects on behavior and organ accumulation. Phytother. Res. 2014, 28, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Riccobono, L.; Spadaro, V.; Campisi, P.; Bruno, M.; Senatore, F. Volatile constituents of the aerial parts of Pulicaria sicula (L.) Moris growing wild in Sicily: Chemotaxonomic volatile markers of the genus Pulicaria Gaertn. Chem. Biodiversity 2015, 12, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.S.; Lajide, L.; Villanueva, H.E.; Setzer, W.N. Essential oil composition and insecticidal activity of Blumea perrottetiana growing in Southwestern Nigeria. Nat. Prod. Commun. 2010, 5, 1135–1138. [Google Scholar] [PubMed] [Green Version]
- Satyal, P.; Chhetri, B.K.; Dosoky, N.S.; Shrestha, S.; Poudel, A.; Setzer, W.N. Chemical composition of Blumea lacera essential oil from Nepal. Biological activities of the essential oil and (Z)-lachnophyllum ester. Nat. Prod. Commun. 2015, 10, 1749–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Huang, M.; Pang, Y.-X.; Yu, F.-L.; Chen, C.; Liu, L.-W.; Chen, Z.-X.; Zhang, Y.-B.; Chen, X.-L.; Hu, X. Variations in essential oil yield, composition, and antioxidant activity of different plant organs from Blumea balsamifera (L.) DC. at different growth times. Molecules 2016, 21, 1024. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Miri, A.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Setzer, W.N.; Hadjiakhoondi, A. Chemical composition and biological activity of Pulicaria vulgaris essential oil from Iran. Nat. Prod. Commun. 2014, 9, 1633–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaib, F.; Allali, H.; Bennaceur, M.; Flamini, G. Chemical composition and antimicrobial activity of essential oils from the aerial parts of Asteriscus graveolens (Forssk.) Less. and Pulicaria incisa (Lam.) DC.: Two Asteraceae herbs growing wild in the Hoggar. Chem. Biodiversity 2017, 14, e1700092. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Muscolo, C.; Zorzetto, C.; Sánchez-Mateo, C.; Rabanal, R.M.; Quassinti, L.; Bramucci, M.; Damiano, S.; Iannarelli, R.; et al. Bioactive secondary metabolites from Schizogyne sericea (Asteraceae) endemic to Canary Islands. Chem. Biodiversity 2016, 13, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Zorzetto, C.; Sánchez-Mateo, C.; Rabanal, R.M.; Iannarelli, R.; Maggi, F. Chemical analysis of the essential oils from Schizogyne sericea growing in different areas of Tenerife (Spain). Biochem. Syst. Ecol. 2016, 65, 192–197. [Google Scholar] [CrossRef]
- Radulović, N.; Blagojević, P.; Palić, R.; Zlatković, B. Volatiles of Telekia speciosa (Schreb.) Baumg. (Asteraceae) from Serbia. J. Essent. Oil Res. 2010, 22, 250–254. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Stojakowska, A.; Kalemba, D. Chemical composition of essential oils from a multiple shoot culture of Telekia speciosa and different plant organs. Nat. Prod. Commun. 2012, 7, 625–628. [Google Scholar] [CrossRef] [Green Version]
- Andreani, S.; De Cian, M.-C.; Paolini, J.; Desjobert, J.-M.; Costa, J.; Muselli, A. Chemical variability and antioxidant activity of Limbarda crithmoides L. essential oil from Corsica. Chem. Biodiversity 2013, 10, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Shtacher, G.; Kashman, Y. Chemical investigation of volatile constituents of Inula viscosa Ait. Tetrahedron 1971, 27, 1343–1349. [Google Scholar] [CrossRef]
- Bokadia, M.M.; MacLeod, A.J.; Mehta, S.C.; Mehta, B.K.; Patel, H. The essential oil of Inula racemosa. Phytochemistry 1986, 25, 2887–2888. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, R.J.; Singh, I.P. Determination of major sesquiterpene lactones in essential oil of Inula racemosa and Saussurea lappa using a qNMR. J. Essent. Oil Bear. Pl. 2016, 19, 20–31. [Google Scholar] [CrossRef]
- Xu, T.; Gherib, M.; Bekhechi, C.; Atik-Bekkara, F.; Casabianca, H.; Tomi, F.; Casanova, J.; Bighelli, A. Thymyl esters derivatives and a new natural product modhephanone from Pulicaria mauritanica Coss. (Asteraceae) root oil. Flavour Fragr. J. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Cilović, E.; Brantner, A.; Tran, H.T.; Arsenijević, J.; Maksimović, Z. Methanol extracts and volatiles of Telekia speciosa (Schreb.) Baumg. from Bosnia and Herzegovina. Technol. Acta 2019, 12, 9–13. [Google Scholar]
- Stojakowska, A.; Michalska, K.; Malarz, J. Simultaneous quantification of eudesmanolides and thymol derivatives from tissues of Inula helenium and I. royleana by reversed-phase high-performance liquid chromatography. Phytochem. Anal. 2006, 17, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Zee, O.P.; Kim, D.K.; Lee, K.R. Thymol derivatives from Carpesium divaricatum. Arch. Pharm. Res. 1998, 21, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Kędzia, B.; Kisiel, W. Antimicrobial activity of 10-isobutyryloxy-8,9-epoxythymol isobutyrate. Fitoterapia 2005, 76, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Kuszewska, K.; Błaszkowski, J.; Sochacka-Obruśnik, A.; Stojakowska, A.; Zubek, S. Associations between root-inhabiting fungi and 40 species of medicinal plants with potential applications in the pharmaceutical and biotechnological industries. Appl. Soil Ecol. 2019, 137, 69–77. [Google Scholar] [CrossRef]
- Bonikowski, R.; Paoli, M.; Szymczak, K.; Krajewska, A.; Wajs-Bonikowska, A.; Tomi, F.; Kalemba, D. Chromatographic and spectral characteristic of some esters of a common monoterpene alcohols. Flav. Fragr. J. 2016, 31, 290–292. [Google Scholar] [CrossRef]
- Talavera-Alemán, A.; Rodríguez-García, G.; López, Y.; García-Gutiérrez, H.A.; Torres-Valencia, J.M.; del Río, R.E.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochem. Rev. 2016, 15, 251–277. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| No | Compound | Amount (%) | RI c exp. | RI d lit. | Identification Method | |||
|---|---|---|---|---|---|---|---|---|
| Aerial Parts | Roots | |||||||
| OF a | G b | OF a | G b | |||||
| 1 | hexanal | - | - | 0.1 | - | 771 | 771 | RI e, MS f |
| 2 | (E)-hex-2-enal | 0.2 | - | - | - | 825 | 832 | RI, MS |
| 3 | hexan-1-ol | 0.1 | - | tr. | - | 852 | 837 | RI, MS |
| 4 | tricyclene | tr. | - | - | - | 917 | 927 | RI, MS |
| 5 | α-thujene | 0.1 | - | - | - | 922 | 932 | RI, MS |
| 6 | α-pinene | 40.2 | 21.8 | 0.1 | 1.8 | 930 | 936 | RI, MS |
| 7 | camphene | 0.3 | - | - | - | 940 | 950 | RI, MS |
| 8 | sabinene | tr. | - | - | - | 944 | 973 | RI, MS |
| 9 | 6-methylhept-5-en-2-one | 0.2 | 0.2 | - | - | 962 | 978 | RI, MS |
| 10 | β-pinene | 0.5 | 0.3 | - | tr. | 966 | 978 | RI, MS |
| 11 | 2-pentylfuran | 0.4 | 0.3 | 0.2 | - | 976 | 981 | RI, MS |
| 12 | trans-2-(pent-2-enyl)furan | 0.1 | 0.1 | - | - | 984 | 984 | RI, MS |
| 13 | α-phellandrene | - | - | tr. | - | 991 | 1002 | RI, MS |
| 14 | δ-car-3-ene | tr. | - | - | - | 1005 | 1010 | RI, MS |
| 15 | m-cymene | 0.2 | tr. | tr. | - | 1006 | 1013 | RI, MS |
| 16 | p-cymene | - | - | tr. | - | 1007 | 1015 | RI, MS |
| 17 | β-phellandrene | - | - | tr. | - | 1014 | 1023 | RI, MS |
| 18 | limonene | 0.2 | tr. | - | 0.1 | 1018 | 1025 | RI, MS |
| 19 | γ-terpinene | 0.1 | 0.2 | - | - | 1047 | 1051 | RI, MS |
| 20 | trans-linalool oxide (furanoid) | tr. | - | tr. | - | 1055 | 1058 | RI, MS |
| 21 | camphen-6-ol | tr. | - | - | - | 1066 | 1082 | RI, MS |
| 22 | terpinolene | tr. | tr. | - | - | 1077 | 1082 | RI, MS |
| 23 | n-nonanal | - | 0.1 | - | - | 1080 | 1076 | RI, MS |
| 24 | linalool | 2.1 | 3.4 | 0.1 | tr. | 1083 | 1086 | RI, MS |
| 25 | 145/89/143/115 M? | 0.1 | - | - | - | 1091 | - | RI, MS |
| 26 | limona ketone | - | - | tr. | - | 1100 | 1105 | RI, MS |
| 27 | α-campholenal | 0.6 | 0.1 | - | - | 1101 | 1105 | RI, MS |
| 28 | cis-p-menth-2-en-1-ol | 0.1 | tr. | 0.1 | 0.1 | 1104 | 1108 | RI, MS |
| 29 | trans-p-menth-2-en-1-ol | - | - | 0.1 | 0.1 | 1119 | 1116 | RI, MS |
| 30 | trans-pinocarveol | 0.7 | 0.2 | - | - | 1120 | 1126 | RI, MS |
| 31 | cis-verbenol | - | tr. | - | - | 1121 | 1132 | RI, MS |
| 32 | trans-verbenol | 0.3 | 0.2 | - | - | 1124 | 1134 | RI, MS |
| 33 | 2-hydroxy-3-methyl- benzaldehyde | - | - | 0.1 | 0.2 | 1126 | 1135 | RI, MS |
| 34 | (E)-non-2-enal | 0.1 | tr. | 0.1 | 0.2 | 1133 | 1136 | RI, MS |
| 35 | nerol oxide | - | - | 0.2 | 1.0 | 1134 | 1137 | RI, MS |
| 36 | pinocarvone | 0.5 | 0.1 | - | - | 1135 | 1137 | RI, MS |
| 37 | p-mentha-1,5-dien-8-ol | 0.1 | 0.2 | - | - | 1143 | 1138 | RI, MS |
| 38 | geijeren | 0.4 | tr. | 4.2 | 3.1 | 1148 | 1139 | RI, MS |
| 39 | terpinen-4-ol | 0.7 | 0.9 | - | - | 1159 | 1164 | RI, MS |
| 40 | myrtenal | - | tr. | - | - | 1165 | 1172 | RI, MS |
| 41 | α-terpineol | 0.8 | 0.7 | 0.1 | 0.1 | 1171 | 1176 | RI, MS |
| 42 | cis-piperitol | - | - | tr. | tr. | 1176 | 1181 | RI, MS |
| 43 | myrtenol | 0.1 | - | - | - | 1177 | 1178 | RI, MS |
| 44 | n-decanal | 0.6 | 1.2 | - | - | 1182 | 1180 | RI, MS |
| 45 | trans-piperitol | - | - | tr. | tr. | 1186 | 1193 | RI, MS |
| 46 | 2-ethenyl-3-methyloanisol | 0.2 | - | 0.7 | 0.9 | 1190 | 1196 | RI, MS |
| 47 | β-cyclocitral | 0.2 | 0.3 | - | - | 1193 | 1195 | RI, MS |
| 48 | trans-carveol | 0.1 | - | - | - | 1195 | 1200 | RI, MS |
| 49 | nerol | 3.7 | 2.1 | 1.4 | 1.2 | 1210 | 1210 | 1H, RI, MS |
| 50 | thymol methyl ether | - | - | 0.4 | 0.1 | 1211 | 1215 | 1H, RI, MS |
| 51 | geraniol | 0.2 | 0.4 | - | - | 1233 | 1235 | RI, MS |
| 52 | α-jonene | 0.1 | - | - | - | 1241 | 1258 | RI, MS |
| 53 | cuminol | tr. | - | 0.3 | 0.3 | 1245 | 1266 | RI, MS |
| 54 | thymol | 0.1 | - | 0.1 | 0.1 | 1258 | 1267 | RI, MS |
| 55 | carvacrol | 0.9 | - | 0.3 | 0.3 | 1264 | 1278 | RI, MS |
| 56 | dihydroedulan II | 0.1 | 0.1 | - | - | 1278 | 1290 | RI, MS |
| 57 | (E,E)-deca-2,4-dienal | 0.1 | - | - | 0.1 | 1286 | 1291 | RI, MS |
| 58 | 4,6-dimethyl-2,3-2H- benzofuran-2-one | - | - | 0.2 | 0.2 | 1317 | - | RI, MS |
| 59 | 7αH-silphiperfol-5-ene | 0.5 | 0.1 | 0.7 | 2.5 | 1323 | 1329 | RI, MS |
| 60 | presilphiperfol-7-ene | 0.2 | - | 0.2 | 0.3 | 1332 | 1342 | RI, MS |
| 61 | 7βH-silphiperfol-5-ene | 0.9 | 0.1 | 1.1 | 3.4 | 1342 | 1352 | RI, MS |
| 62 | α-cubebene | tr. | 0.1 | - | - | 1344 | 1355 | RI, MS |
| 63 | α-longipinene | 0.2 | tr. | 0.3 | 0.4 | 1348 | 1360 | RI, MS |
| 64 | (E)-tridec-6-en-4-yn | 0.2 | 0.1 | - | - | 1363 | - | RI, MS |
| 65 | viburtinal | - | - | 0.5 | 1.2 | 1367 | - | RI, MS |
| 66 | longicyclene | 0.4 | - | 0.3 | 0.4 | 1371 | 1372 | RI, MS |
| 67 | cyclosativene | 0.4 | - | - | - | 1372 | 1378 | RI, MS |
| 68 | α-copaene | - | 0.5 | - | - | 1375 | 1379 | RI, MS |
| 69 | silphiperfol-6-ene | - | - | 0.3 | 0.4 | 1376 | 1379 | RI, MS |
| 70 | modephene | 0.2 | - | 0.3 | 1.0 | 1377 | 1383 | RI, MS |
| 71 | α-isocomene | 0.4 | 0.3 | 0.4 | 1.3 | 1383 | 1389 | RI, MS |
| 72 | 137/121/95/136 M204 | 0.6 | 0.1 | 0.5 | 0.8 | 1391 | - | RI, MS |
| 73 | 6-methoxythymol methyl-ether | 2.1 | 0.5 | 2.7 | 1.4 | 1394 | 1398 | RI, MS |
| 74 | β-isocomene | 0.6 | 0.2 | 0.7 | 2.0 | 1402 | 1411 | RI, MS |
| 75 | α-cedrene | 0.1 | - | tr. | tr. | 1409 | 1418 | RI, MS |
| 76 | α-gurjunene | - | tr. | - | - | 1410 | 1418 | RI, MS |
| 77 | α-santalene | 1.0 | 0.5 | 0.6 | 0.8 | 1413 | 1422 | RI, MS |
| 78 | trans-geranylacetone | 0.3 | 0.3 | - | - | 1423 | 1430 | RI, MS |
| 79 | trans-α-bergamotene | 0.6 | 0.1 | 0.3 | 0.2 | 1428 | 1434 | RI, MS |
| 80 | epi-β-santalene | 1.5 | 0.4 | 0.9 | 0.8 | 1438 | 1446 | RI, MS |
| 81 | α-himachalene | 0.5 | 0.1 | - | - | 1441 | 1450 | RI, MS |
| 82 | aromadendrene | 0.2 | - | 0.3 | 0.4 | 1442 | 1449 | RI, MS |
| 83 | α-humulene | 0.1 | - | tr. | tr. | 1446 | 1455 | RI, MS |
| 84 | 8,9-didehydrothymyl- isobutyrate | 0.9 | 0.2 | 1.6 | 0.8 | 1461 | 1458 | RI, MS |
| 85 | thymyl-isobutyrate | 2.0 | 1.0 | 6.3 | 3.5 | 1467 | 1462 | 1H, RI, MS |
| 86 | β-jonone | 0.9 | 1.5 | - | - | 1468 | 1468 | RI, MS |
| 87 | neryl isobutyrate | 3.2 | 3.9 | 4.1 | 3.9 | 1475 | 1468 | 1H, RI, MS |
| 88 | γ-himachalene | 0.3 | 0.1 | - | - | 1480 | 1479 | RI, MS |
| 89 | 123/93/94/121 M204 | 0.8 | 0.1 | 0.5 | 0.7 | 1484 | - | RI, MS |
| 90 | (3E,6Z)-α-farnesene | 1.8 | 5.5 | - | - | 1487 | 1475 | RI, MS |
| 91 | α-terpinyl isovalerate | - | - | 0.2 | 0.7 | 1489 | 1488 | RI, MS |
| 92 | γ-muurolene | tr. | 0.2 | - | - | 1493 | 1494 | RI, MS |
| 93 | elixene (4-isopropylidene-1-vinyl-o- menth-8-ene) | - | - | 0.2 | 0.2 | 1498 | 1493 | RI, MS |
| 94 | ledene | 1.5 | 2.2 | tr. | 0.2 | 1499 | 1491 | RI, MS |
| 95 | α-muurolene | - | 1.1 | - | - | 1500 | 1496 | RI, MS |
| 96 | (E,E)-α-farnesene | 0.7 | 1.1 | - | - | 1502 | 1498 | RI, MS |
| 97 | β-bisabolene | 1.6 | 0.6 | 0.5 | 0.4 | 1507 | 1503 | RI, MS |
| 98 | γ-cadinene | 1.1 | 3.2 | - | - | 1511 | 1507 | RI, MS |
| 99 | cameronan-7α-ol | - | - | tr. | 0.1 | 1513 | 1513 | RI, MS |
| 100 | α-photosantalol | - | - | 0.1 | 0.2 | 1514 | 1514 | RI, MS |
| 101 | isolongifolan-8-ol | - | - | 0.1 | 0.5 | 1517 | 1515 | RI, MS |
| 102 | cis/trans-calamenene | 0.2 | 0.4 | 0.2 | 0.3 | 1526 | 1517 | RI, MS |
| 103 | δ-cadinene | 1.4 | 5.3 | - | - | 1520 | 1520 | RI, MS |
| 104 | β-cadinene | 0.1 | 0.3 | - | - | 1523 | 1526 | RI, MS |
| 105 | 9-methoxycalamenene | 0.1 | - | - | - | 1524 | - | RI, MS |
| 106 | 147/162/121/177 M206 | - | - | tr. | 0.1 | 1531 | - | RI, MS |
| 107 | 121/163/93/134 M218 | - | - | 0.2 | 0.1 | 1534 | - | RI, MS |
| 108 | α-cadinene | 0.3 | 0.5 | - | - | 1535 | 1534 | RI, MS |
| 109 | (E)-α-bisabolene | 0.1 | 0.2 | 0.1 | 0.7 | 1536 | 1530 | RI, MS |
| 110 | (E)-nerolidol | 2.2 | 8.6 | 0.6 | 0.6 | 1543 | 1553 | 1H, RI, MS |
| 111 | thymyl-2-methylbutyrate | 0.1 | - | 0.2 | 0.2 | 1546 | - | RI, MS |
| 112 | neryl-α-methylbutyrate | 1.7 | 1.6 | 3.6 | 3.4 | 1551 | 1565 | RI, MS |
| 113 | neryl isovalerate | 1.6 | 1.3 | 2.3 | 1.8 | 1557 | 1579 | RI, MS |
| 114 | caryophyllene oxide | 0.4 | 0.4 | 1.1 | 2.1 | 1565 | 1578 | 1H, RI, MS |
| 115 | viridiflorol | 0.5 | 1.4 | - | - | 1577 | 1592 | RI, MS |
| 116 | isoaromadendreneepoxide | 0.3 | 0.1 | - | - | 1584 | 1590 | RI, MS |
| 117 | ledol | 0.3 | 0.5 | - | - | 1588 | 1600 | RI, MS |
| 118 | humulene II epoxide | - | - | 0.4 | 1.1 | 1595 | 1602 | RI, MS |
| 119 | 1,10-di-epi-cubenol | 0.1 | 0.2 | - | - | 1597 | 1615 | RI, MS |
| 120 | 135/146/159/71 M218 | - | - | 0.2 | 0.3 | 1602 | - | RI, MS |
| 121 | muurola-4,10(14)-dien-1β-ol | 0.2 | 0.2 | - | - | 1605 | 1620 | RI, MS |
| 122 | gossonorol | - | - | 0.1 | 0.1 | 1613 | 1626 | RI, MS |
| 123 | 1-epi-cubenol | 0.2 | 0.5 | - | - | 1614 | 1623 | RI, MS |
| 124 | α-acorenol | tr. | - | 0.4 | 0.5 | 1620 | 1623 | RI, MS |
| 125 | τ-cadinol | 1.4 | 4.1 | - | - | 1625 | 1633 | 1H, RI, MS |
| 126 | τ-muurolol | 0.3 | 0.4 | - | - | 1628 | 1633 | RI, MS |
| 127 | β-eudesmol | 0.6 | 0.2 | 3.4 | 3.8 | 1631 | 1641 | RI, MS |
| 128 | α-cadinol | 1.3 | 3.8 | 0.4 | 0.3 | 1638 | 1643 | 1H, RI, MS |
| 129 | 5β,7βH,10α-eudesm-11-en- 1α-ol | - | - | 0.3 | - | 1653 | - | RI, MS |
| 130 | 6-methoxythymyl isobutyrate | 0.9 | 0.7 | 3.8 | 4.9 | 1657 | 1658 | 1H,13C, RI, MS |
| 131 | 6-methoxy-8,9-didehydrothymyl isobutyrate | tr. | - | 0.4 | 0.2 | 1665 | 1676 | RI, MS |
| 132 | 10-isobutyryloxy-8,9- didehydrothymol-methyl-ether | - | - | 0.4 | 0.3 | 1666 | 1684 | 1H,13C, RI, MS |
| 133 | α-bisabolol | 0.1 | 0.4 | 0.3 | 1.3 | 1668 | 1683 | RI, MS |
| 134 | 145/162/71/115 M232 | - | - | 0.3 | 0.5 | 1681 | - | RI, MS |
| 135 | aromadendrene oxide | 0.1 | 0.1 | - | - | 1702 | 1672 | RI, MS |
| 136 | 135/148/133/91 M236 | - | - | 0.1 | 0.1 | 1725 | - | RI, MS |
| 137 | 135/164/71/91 M234 | - | - | 0.2 | 0.1 | 1733 | - | RI, MS |
| 138 | fenantrene (artifact) | 0.1 | 0.3 | - | - | 1741 | 1744 | RI, MS |
| 139 | diisobutylphtalate (artifact) | 0.9 | 2.5 | 0.3 | 0.6 | 1817 | 1819 | RI, MS |
| 140 | hexahydrofarnesylacetone | 0.2 | 0.8 | - | - | 1820 | 1830 | RI, MS |
| 141 | alantolactone | 0.1 | - | 0.2 | tr. | 1854 | 1878 | RI, MS |
| 142 | 9-isobutyryloxythymyl- isobutyrate | 0.2 | 0.8 | 5.6 | 5.7 | 1879 | 1891 | 1H,13C, RI, MS |
| 143 | 10-isobutyryloxy-8,9- didehydrothymyl-isobutyrate | - | - | 3.0 | 3.1 | 1882 | 1891 | RI, MS |
| 144 | (5E,9E)-farnesylacetone | 0.1 | 0.2 | - | - | 1889 | 1895 | RI, MS |
| 145 | dibutylphtalate (artifact) | 0.2 | 1.8 | - | - | 1906 | 1909 | RI, MS |
| 146 | 7-isobutyryloxythymyl- isobutyrate | - | - | 0.6 | 0.7 | 1914 | 1924 | RI, MS |
| 147 | 9-(2-methylbutyryloxy)thymyl-isobutyrate | - | - | 1.0 | 1.4 | 1964 | 1970 | RI, MS |
| 148 | 10-(2-methylbutyryloxy)-8,9- didehydrothymyl-isobutyrate | 0.1 | 0.4 | 0.3 | 0.4 | 1967 | 1970 | RI, MS |
| 149 | 10-isobutyryloxy-8,9- epoxythymyl-isobutyrate | 0.2 | 0.6 | 29.2 | 18.1 | 2002 | 2036 | 1H,13C,RI,MS |
| 150 | 71/177/150/135 M290 | - | - | 0.9 | 0.5 | 2048 | - | RI, MS |
| 151 | 10-(2-methylbutyryloxy)-8,9- epoxythymyl-isobutyrate | - | - | 4.4 | 3.6 | 2077 | 2056 | RI, MS |
| 152 | 10-isovaleroxy-8,9- epoxythymyl-isobutyrate | - | - | 0.3 | 0.1 | 2097 | 2122 | RI, MS |
| 153 | fitol | 0.3 | 1.7 | - | - | 2098 | - | RI, MS |
| 154 | 57/177/71/85 M304 | - | - | 0.1 | 0.1 | 2149 | - | RI, MS |
| 155 | tricosane | 0.1 | 0.2 | - | - | 2286 | 2300 | RI, MS |
| 156 | tetracosane | 0.1 | - | - | - | 2386 | 2400 | RI, MS |
| 157 | pentacosane | 0.6 | 0.3 | - | - | 2489 | 2500 | RI, MS |
| 158 | hexacosane | tr. | - | - | - | 2589 | 2600 | RI, MS |
| 159 | heptacosane | 0.2 | 0.1 | - | - | 2685 | 2700 | RI, MS |
| Sum of Identified | 96.7 | 97.5 | 94.3 | 91.7 | ||||
| Yield of Essential Oil (%) | 0.016 | 0.014 | 0.150 | 0.059 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajs-Bonikowska, A.; Malarz, J.; Stojakowska, A. Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland. Molecules 2019, 24, 4418. https://doi.org/10.3390/molecules24234418
Wajs-Bonikowska A, Malarz J, Stojakowska A. Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland. Molecules. 2019; 24(23):4418. https://doi.org/10.3390/molecules24234418
Chicago/Turabian StyleWajs-Bonikowska, Anna, Janusz Malarz, and Anna Stojakowska. 2019. "Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland" Molecules 24, no. 23: 4418. https://doi.org/10.3390/molecules24234418
APA StyleWajs-Bonikowska, A., Malarz, J., & Stojakowska, A. (2019). Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland. Molecules, 24(23), 4418. https://doi.org/10.3390/molecules24234418