An Enantioselective Potentiometric Sensor for 2-Amino-1-Butanol Based on Chiral Porous Organic Cage CC3-R
Abstract
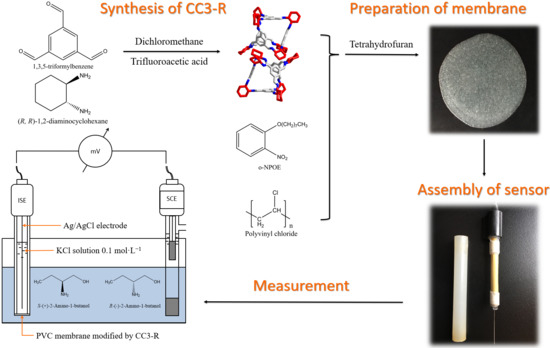
:1. Introduction
2. Results and Discussion
2.1. Characterisation of the Synthesised CC3-R
2.2. Optimisation of Membrane Components
2.3. Effect of pH on the Electrode
2.4. Enantioselectivity Coefficient of the Electrode
2.5. Recognition of Mixing Samples
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CC3-R
3.3. Preparation of Enantioselective Membrane Electrodes
3.4. Potentiometric Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duan, A.H.; Wang, B.J.; Xie, S.M.; Zhang, J.H.; Yuan, L.M. A chiral, porous, organic cage-based, enantioselective potentiometric sensor for 2-aminobutanol. Chirality 2017, 29, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, Y.Y.; Wang, Y.Q.; Gao, J.Z. Chiral salen Mn(III) complex-based enantioselective potentiometric sensor for L-mandelic acid. Anal. Chim. Acta 2009, 653, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Bi, R.L.; Xu, L.; Liu, Y. A potentiometric chiral sensor for l-Phenylalanine based on crosslinked polymethylacrylic acid–polycarbazole hybrid molecularly imprinted polymer. Anal. Chim. Acta 2012, 754, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van, S.R.I.; Nhlapo, N.S.; Staden-van, J.F.; Aboul-Enein, H.Y. Enantioanalysis of (-)butaclamol using vancomycin and teicoplanin as chiral Selectors. Comb. Chem. High Throughput Screen. 2010, 13, 690–693. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Nagaoka, T.; Yu, B.; Levon, K. Chiral ligand exchange potentiometric aspartic acid sensors with polysiloxane films containing a chiral ligand N-carbobenzoxy-aspartic acid. Anal. Chem. 2009, 81, 1888–1892. [Google Scholar] [CrossRef]
- Stefan-van, S.R.I.; Staden-van, J.F.; Aboul-Enein, H.Y. Macrocyclic antibiotics as chiral selectors in the design of enantioselective, potentiometric membrane electrodes for the determination of S-flurbiprofen. Anal. Bioanal. Chem. 2009, 394, 821–826. [Google Scholar] [CrossRef]
- Yin, X.L.; Ding, J.J.; Zhang, S.; Kong, J.L. Enantioselective sensing of chiral amino acids by potentiometric sensors based on optical active polyaniline films. Biosens. Bioelectron. 2006, 21, 2184–2187. [Google Scholar] [CrossRef]
- Ozoemena, K.I.; Stefan, R.I. Enantioselective potentiometric membrane electrodes based on α-, β- and γ-cyclodextrins as chiral selectors for the assay of l-proline. Talanta 2005, 66, 501–504. [Google Scholar] [CrossRef]
- Rat’ko, A.A.; Stefan, R.I.; Staden-van, J.F.; Aboul-Enein, H.Y. Macrocyclic antibiotics as chiral selectors in the design of enantioselective, potentiometric membrane electrodes for the determination of L- and D-enantiomers of methotrexate. Talanta 2004, 64, 145–150. [Google Scholar] [CrossRef]
- Hui, M.D.; Yang, Y.H.; Li, B.J.; Pan, F.; Zhu, G.Z.; Zeller, M.; Yuan, D.Q.; Wang, C. Targeted synthesis of a large tria zine-based [4+6] organic molecular cage: Structure, porosity and gas separation. Chem. Commun. 2015, 51, 1976–1979. [Google Scholar]
- Zhang, G.; Presly, O.; White, F.; Oppel, I.M.; Mastalerz, M. A permanent mesoporous organic cage with an exceptionally high surface area. Angew. Chem. Int. Ed. 2014, 53, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Mastalerz, M. Organic cage compounds—From shape-persistency to function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Little, M.A.; Jelfs, K.E.; Jones, J.T.A.; Schmidtmann, M.; Chong, S.Y.; Hasell, T.; Cooper, A.I. Acid- and base-stable porous organic cages: Shape persistence and pH stability via post-synthetic “tying” of a flexible amine cage. J. Am. Chem. Soc. 2014, 136, 7583–7586. [Google Scholar] [CrossRef] [PubMed]
- Hasell, T.; Miklitz, M.; Stephenson, A.; Little, M.A.; Chong, S.Y.; Clowes, R.; Chen, L.J.; Holden, D.; Tribello, G.A.; Jelfs, K.E.; et al. Porous organic cages for sulfur hexafluoride separation. J. Am. Chem. Soc. 2016, 138, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M.; Schneider, M.W.; Oppel, I.M.; Presly, O. A salicylbisimine cage compound with high surface area and selective CO2/CH4 adsorption. Angew. Chem. Int. Ed. 2011, 50, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Voss, B.A.; Jin, A.; Long, H.; Noble, R.D.; Zhang, W. Highly CO2-selective organic molecular cages: What determines the CO2 selectivity. J. Am. Chem. Soc. 2011, 133, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Wu, X.F.; Clowes, R.; Jones, J.T.A.; Jelfs, K.E.; Adams, D.J.; Trewin, A.; Bacsa, J.; Steiner, A.; Cooper, A.I. A soft porous organic cage crystal with complex gas sorption behavior. Chem. Eur. J. 2011, 17, 10235–10240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Xie, S.M.; Wang, B.J.; He, P.G.; Yuan, L.M. A homochiral porous organic cage with large cavity and pore windows for the efficient gas chromatography separation of enantiomers and positional isomers. J. Sep. Sci. 2018, 41, 1385–1394. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhu, P.J.; Xie, S.M.; Zi, M.; Yuan, L.M. Homochiral porous organic cage used as stationary phase for open tubular capillary electrochromatography. Anal. Chim. Acta 2018, 999, 169–175. [Google Scholar] [CrossRef]
- Song, Q.L.; Jiang, S.; Hasell, T.; Liu, M.; Sun, S.J.; Cheetham, A.K.; Sivaniah, E.; Cooper, A.I. Porous organic cage thin films and molecular-sieving membranes. Adv. Mater. 2016, 28, 2629–2637. [Google Scholar] [CrossRef]
- Xie, S.M.; Zhang, J.H.; Fu, N.; Wang, B.J.; Hu, C.; Yuan, L.M. Application of homochiral alkylated organic cages as chiral stationary phases for molecular separations by capillary gas chromatography. Molecules 2016, 21, 1466. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.M.; Zhang, J.H.; Fu, N.; Wang, B.J.; Chen, L.; Yuan, L.M. A chiral porous organic cage for molecular recognition using gas chromatography. Anal. Chim. Acta 2016, 903, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Xie, S.M.; Wang, B.J.; He, P.G.; Yuan, L.M. Highly selective separation of enantiomers using a chiral porous organic cage. J. Chromatogr. A 2015, 1426, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kewley, A.; Stephenson, A.; Chen, L.J.; Briggs, M.E.; Hasell, T.; Cooper, A.I. Porous organic cages for gas chromatography separations. Chem. Mater. 2015, 27, 3207–3210. [Google Scholar] [CrossRef]
- Mitra, T.; Jelfs, K.E.; Schmidtmann, M.; Ahmed, A.; Chong, S.Y.; Adams, D.J.; Cooper, A.I. Molecular shape sorting using molecular organic cages. Nat. Chem. 2013, 5, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Reiss, P.S.; Chong, S.Y.; Holden, D.; Jelfs, K.E.; Hasell, T.; Little, M.A.; Kewley, A.; Briggs, M.E.; Stephenson, A.; et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages. Nat. Mater. 2014, 13, 954–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.M.; Yuan, L.M. Recent progress of chiral stationary phases for separation of enantiomers in gas chromatography. J. Sep. Sci. 2017, 40, 124–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Ge, J.; Lin, R.; Chen, C.; Peng, Q.; Wang, D.S.; Li, Y.D. Porous organic cage stabilised palladium nanoparticles: Efficient heterogeneous catalysts for carbonylation reaction of aryl halides. Chem. Commun. 2018, 54, 2796–2799. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, J.W. Amorphous porous organic cage membranes for water desalination. J. Phys. Chem. C 2018, 122, 1732–1740. [Google Scholar] [CrossRef]
- Brutschy, M.; Schneider, M.W.; Mastalerz, M.; Waldvogel, S.R. Porous organic cage compounds as highly potent affinity materials for sensing by quartz crystal microbalances. Adv. Mater. 2012, 24, 6049–6052. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Briggs, M.; Jones, J.T.A.; Adams, D.J.; Chong, S.Y.; Schmidtmann, M.; Cooper, A.I. Supramolecular engineering of intrinsic and extrinsic porosity in covalent organic cages. J. Am. Chem. Soc. 2011, 133, 16566–16571. [Google Scholar] [CrossRef] [PubMed]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Yashima, E.; Okamoto, Y. Structural analysis of amylose tris(3,5-dimethylphenylcarbamate) by NMR relevant to its chiral recognition mechanism in HPLC. J. Am. Chem. Soc. 2002, 124, 12583–12589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Xie, S.M.; Chen, L.; Wang, B.J.; He, P.G.; Yuan, L.M. Homochiral porous organic cage with high selectivity for the separation of racemates in gas chromatography. Anal. Chem. 2015, 87, 7817–7824. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.S.B.; Da-Silva, E.T.; De-Souza, M.V.N. An environmentally friendly, scalable and highly efficient synthesis of (S,S)-Ethambutol, a first line drug against tuberculosis. Lett. Org. Chem. 2015, 12, 478–481. [Google Scholar] [CrossRef]
- Dobrikov, G.M.; Valcheva, V.; Stoilova-Disheva, M.; Momekov, G.; Tzvetkova, P.; Chimov, A.; Dimitrov, V. Synthesis and in vitro antimycobacterial activity of compounds derived from (R)- and (S)-2-amino-1-butanol—The crucial role of the configuration. Eur. J. Med. Chem. 2012, 48, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Dobrikov, G.M.; Valcheva, V.; Nikolova, Y.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Efficient synthesis of new (R)-2-amino-1-butanol derived ureas, thioureas and acylthioureas and in vitro evaluation of their antimycobacterial activity. Eur. J. Med. Chem. 2013, 63, 468–473. [Google Scholar] [CrossRef]
- Dobrikov, G.M.; Valcheva, V.; Nikolova, Y.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Enantiopure antituberculosis candidates synthesized from (-)-fenchone. Eur. J. Med. Chem. 2014, 77, 243–247. [Google Scholar] [CrossRef]
- Horváth, V.; Takécs, T.; Horvai, G.; Huszthy, P.; Bradshaw, J.S.; Izatt, R.M. Enantiomer-Selectivity of ion-selective electrodes based on a chiral crown-ether ionophore. Anal. Lett. 1997, 9, 1591–1609. [Google Scholar] [CrossRef]
- Moody, G.J.; Oke, R.B.; Thomas, J.D.R. A calcium-sensitive electrode based on a liquid ion exchanger in a poly(vinyl chloride) matrix. Analyst 1970, 95, 910–918. [Google Scholar] [CrossRef]
Sample Availability: Not available. |








| Interference Ion | |
|---|---|
| R-2-Amino-1-butanol | −0.98 |
| S-2-Amino-3-phenyl-1-propanol | −0.59 |
| R-2-Amino-3-phenyl-1-propanol | −0.59 |
| S-2-Amino-3-methyl-1-butanol | 0.31 |
| R-2-Amino-3-methyl-1-butanol | 0.26 |
| S-3-Amino-1,2-propanediol | −0.41 |
| R-3-Amino-1,2-propanediol | −0.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-J.; Duan, A.-H.; Zhang, J.-H.; Xie, S.-M.; Cao, Q.-E.; Yuan, L.-M. An Enantioselective Potentiometric Sensor for 2-Amino-1-Butanol Based on Chiral Porous Organic Cage CC3-R. Molecules 2019, 24, 420. https://doi.org/10.3390/molecules24030420
Wang B-J, Duan A-H, Zhang J-H, Xie S-M, Cao Q-E, Yuan L-M. An Enantioselective Potentiometric Sensor for 2-Amino-1-Butanol Based on Chiral Porous Organic Cage CC3-R. Molecules. 2019; 24(3):420. https://doi.org/10.3390/molecules24030420
Chicago/Turabian StyleWang, Bang-Jin, Ai-Hong Duan, Jun-Hui Zhang, Sheng-Ming Xie, Qiu-E Cao, and Li-Ming Yuan. 2019. "An Enantioselective Potentiometric Sensor for 2-Amino-1-Butanol Based on Chiral Porous Organic Cage CC3-R" Molecules 24, no. 3: 420. https://doi.org/10.3390/molecules24030420
APA StyleWang, B. -J., Duan, A. -H., Zhang, J. -H., Xie, S. -M., Cao, Q. -E., & Yuan, L. -M. (2019). An Enantioselective Potentiometric Sensor for 2-Amino-1-Butanol Based on Chiral Porous Organic Cage CC3-R. Molecules, 24(3), 420. https://doi.org/10.3390/molecules24030420