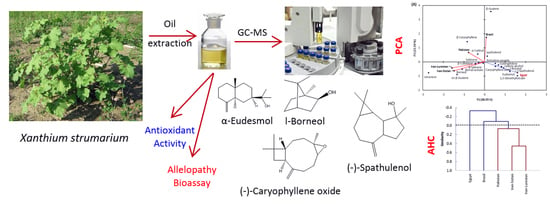
Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium Strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Constituents of EO of the Leaves of X. Strumarium
2.2. Principal Components Analysis (PCA) and Agglomerative Hierarchical Clustering (AHC)
2.3. Allelopathic Activity
2.4. Antioxidant Activity
3. Material and Methods
3.1. Plant Material
3.2. Extraction of EO
3.3. Identification of EO Constituents
3.4. Allelopathic Activity
3.5. Antioxidant Activity
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baratta, M.T.; Dorman, H.D.; Deans, S.G.; Biondi, D.M.; Ruberto, G. Chemical composition, antimicrobial and antioxidative activity of laurel, sage, rosemary, oregano and coriander essential oils. J. Essent. Oil Res. 1998, 10, 618–627. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.S.; Ismail, S.; Man, C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012, 92, 593–597. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1636. [Google Scholar] [CrossRef]
- Weston, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; El Gendy, A.; Elshamy, A.; Omer, E. Chemical composition of the essential oil of Trianthema portulacastrum L. Aerial parts and potential antimicrobial and phytotoxic activities of its extract. J. Essent. Oil Bear. Pl. 2016, 19, 1684–1692. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crop. Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Ens, E.J.; Bremner, J.B.; French, K.; Korth, J. Identification of volatile compounds released by roots of an invasive plant, bitou bush (Chrysanthemoides monilifera spp. rotundata), and their inhibition of native seedling growth. Biol. Invas. 2009, 11, 275–287. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Smaeili, S.; Merikhi, M. Essential oil analysis and phytotoxic activity of two ecotypes of Zataria multiflora Boiss. growing in Iran. Nat. Prod. Res. 2010, 24, 1598–1609. [Google Scholar] [CrossRef]
- Joshi, S.; Rojatkar, S.; Nagasampagi, B. Antimalarial activity of Xanthium strumarium. J. Med. Aromat. Pl. Sci. 1997, 19, 366–368. [Google Scholar]
- Kinghorn, A.; Farnsworth, N.; Soejarto, D.; Cordell, G.; Swanson, S.; Pezzuto, J.; Wani, M.; Wall, M.; Oberlies, N.; Kroll, D. Novel strategies for the discovery of plant-derived anticancer agents. Pharm. Biol. 2003, 41, 53–67. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K. Phytopharmacological review of Xanthium strumarium L.(Cocklebur). Int. J. Green Pharm. 2010, 4, 129–139. [Google Scholar] [CrossRef]
- Ginesta-Peris, E.; Garcia-Breijo, F.-J.; Primo-Yúfera, E. Antimicrobial activity of xanthatin from Xanthium spinosum L. Letters in Applied Microbiology 1994, 18, 206–208. [Google Scholar] [CrossRef]
- Tsankova, E.T.; Trendafilova, A.B.; Kujumgiev, A.I.; Galabov, A.S.; Robeva, P.R. Xanthanolides of Xanthium italicum Moretti and their biological activity. Z. Naturforsch. C 1994, 49, 154–156. [Google Scholar] [CrossRef]
- Talakal, T.; Dwivedi, S.; Sharma, S. In vitro and in vivo antitrypanosomal activity of Xanthium strumarium leaves. J. Ethnopharmacol. 1995, 49, 141–145. [Google Scholar] [CrossRef]
- Qin, L.; Han, T.; Li, H.; Zhang, Q.; Zheng, H. A new thiazinedione from Xanthium strumarium. Fitoterapia 2006, 77, 245–246. [Google Scholar] [CrossRef]
- Han, T.; Li, H.-L.; Zhang, Q.-Y.; Han, P.; Zheng, H.-C.; Rahman, K.; Qin, L.-P. Bioactivity-guided fractionation for anti-inflammatory and analgesic properties and constituents of Xanthium strumarium L. Phytomedicine 2007, 14, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Lim, H.J.; Lee, H.J.; Kim, H.-D.; Jeon, R.; Ryu, J.H. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by xanthanolides isolated from Xanthium strumarium. Bioorg. Med. Chem. Lett. 2008, 18, 2179–2182. [Google Scholar] [CrossRef]
- Shah, F.; Ahmad, N.; Zahid, D.; Masood, K.; Ahmad, S. The hudiara drain waste water effect on the distribution of surrounding herbaceous vegetation. Pak. J. Bot. 2010, 42, 1745–1754. [Google Scholar]
- Yadava, R.; Jharbade, J. Novel biologically active triterpenoid saponin from the leaves of Xanthium strumarium Linn. Asian J. Chem. 2007, 19, 1224–1230. [Google Scholar]
- Sheu, S.J.; Hsu, F.L.; Tai, H.M.; Sheu, M.J.; Huang, M.H. Determination of xanthii constituents by high-performance liquid chromatography and capillary electrophoresis. J. Food Drug Anal. 2003, 11, 67–71. [Google Scholar]
- Saxena, V.; MISHRA, M. Xanthanolides from Xanthium strumarium. Fitoterapia 1995, 66, 159–161. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rustaiyan, A.; Akbari, M.T.; Moazami, N.; Masoudi, S.; Amiri, H. Composition of the essential oils of Xanthium strumarium L. and Cetaurea solstitialis L. from Iran. J. Essent. Oil Res. 2006, 18, 427–429. [Google Scholar] [CrossRef]
- Parveen, Z.; Mazhar, S.; Siddique, S.; Manzoor, A.; Ali, Z. Chemical composition and antifungal activity of essential oil from Xanthium strumarium L. leaves. Indian J. Pharm. Sci. 2017, 79, 316–321. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, R.; Raeisi, S. Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L. Molecules 2015, 20, 7034–7047. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.T.d.S.; Gonçalez, E.; Felicio, R.C.; Felicio, J. Evaluation of antifungal activity of Pittosporum undulatum L. essential oil against Aspergillus flavus and aflatoxin production. Cienc. Agrotec. 2011, 35, 71–76. [Google Scholar] [CrossRef]
- Khazaie, H.R.; Nadjafi, F.; Bannayan, M. Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis). Ind. Crop. Prod. 2008, 27, 315–321. [Google Scholar] [CrossRef]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.; Figueiredo, A.; Barroso, J.; Pedro, L. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Costa, L.C.B.; Pinto, J.E.B.P.; Bertolucci, S.K.V.; Costa, J.C.d.B.; Alves, P.B.; Niculau, E.d.S. In vitro antifungal activity of Ocimum selloi essential oil and methylchavicol against phytopathogenic fungi. Rev. Cienc. Agron. 2015, 46, 428–435. [Google Scholar] [CrossRef]
- Li, Z.J.; Njateng, G.S.; He, W.J.; Zhang, H.X.; Gu, J.L.; Chen, S.N.; Du, Z.Z. Chemical composition and antimicrobial activity of the essential oil from the edible aromatic plant Aristolochia delavay. Chem. Biodivers. 2013, 10, 2032–2041. [Google Scholar] [CrossRef]
- Scherer, R.; Wagner, R.; Meireles, M.; Godoy, H.; Duarte, M.; Filho, J. Biological activity and chemical composition of hydrodistilled and supercritical extracts of Xanthium strumarium L. leaves. J. Essent. Oil Res. 2010, 22, 424–429. [Google Scholar] [CrossRef]
- Andrade, M.A.; das Graças Cardoso, M.; de Andrade, J.; Silva, L.F.; Teixeira, M.L.; Valério Resende, J.M.; da Silva Figueiredo, A.C.; Barroso, J.G. Chemical composition and antioxidant activity of essential oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, B.; Loo, J.; Gaisberger, H.; van Zonneveld, M.J.; Schueler, S.; Konrad, H.; Kadu, C.A.; Geburek, T. Conservation priorities for Prunus africana defined with the aid of spatial analysis of genetic data and climatic variables. PLoS ONE 2013, 8, e59987. [Google Scholar] [CrossRef]
- Pérez-Sánchez, R.; Gálvez, C.; Ubera, J.L. Bioclimatic influence on essential oil composition in South Iberian Peninsular populations of Thymus zygis. J. Essent. Oil Res. 2012, 24, 71–81. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers. 2018, 15, e1800392. [Google Scholar] [CrossRef]
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Macías, F.A.; Galindo, J.C.G.; Massanet, G.M. Potential allelopathic activity of several sesquiterpene lactone models. Phytochemistry 1992, 31, 1969–1977. [Google Scholar] [CrossRef]
- Paul, V.J.; Cronan, J.M.; Cardellina, J.H. Isolation of new brominated sesquiterpene feeding deterrents from tropical green alga Neomeris annulata (Dasycladaceae: Chlorophyta). J. Chem. Ecol. 1993, 19, 1847–1860. [Google Scholar] [CrossRef]
- Demirci, F.; Guven, K.; Demirci, B.; Dadandi, M.; Baser, K. Antibacterial activity of two Phlomis essential oils against food pathogens. Food Control 2008, 19, 1159–1164. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M.; El-Amier, Y.A.; Bonanomi, G. Allelopathic activity and chemical composition of Rhynchosia minima (L.) DC. essential oil from Egypt. Chem. Biodivers. 2018, 15, e1700438. [Google Scholar] [CrossRef] [PubMed]
- Oster, U.; Spraul, M.; Rüdiger, W. Natural inhibitors of germination and growth, V possible allelopathic effects of compounds from Thuja occidentalis. Z. Naturforschung C 1990, 45, 835–844. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Mata, A.; Proença, C.; Ferreira, A.; Serralheiro, M.; Nogueira, J.; Araújo, M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chemistry 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| No | RT a | KI b | KI c | Compound Name | Conc. (%) d |
|---|---|---|---|---|---|
| Non-oxygenated Monoterpenoids | |||||
| 1 | 29.32 | 1341 | 1343.4 | 1,5-Dimethyltetralin | 14.27 ± 0.04 |
| Oxygenated Monoterpenoids | |||||
| 2 | 7.93 | 1346 | 1341.4 | α-Terpinyl acetate | 0.56 ± 0.01 |
| 3 | 13.78 | 1165 | 1161.9 | l-Borneol | 6.59 ± 0.02 |
| 4 | 18.44 | 1285 | 1266.1 | L-bornyl acetate | 3.77 ± 0.03 |
| Non-oxygenated sesquiterpenoids | |||||
| 5 | 20.86 | 1374 | 1319.9 | α-Copaene | 0.34 ± 0.01 |
| 6 | 22.08 | 1351 | 1347.1 | α-Cubebene | 0.57 ± 0.01 |
| 7 | 22.62 | 1431 | 1359.2 | β-Copaene | 0.39 ± 0.01 |
| 8 | 23.91 | 1415 | 1388 | Caryophyllene | 0.91 ± 0.02 |
| 9 | 26.27 | 1476 | 1442.2 | γ-Muurolene | 0.56 ± 0.01 |
| 10 | 26.49 | 1482 | 1447.4 | Germacrene-D | 0.75 ± 0.01 |
| 11 | 26.86 | 1492 | 1455.8 | β-Selinene | 1.16 ± 0.02 |
| 12 | 26.97 | 1499 | 1458.6 | β-Guaiene | 0.34 ± 0.01 |
| 13 | 28.02 | 1513 | 1483.8 | β-Cadinene | 1.36 ± 0.03 |
| 14 | 28.28 | 1517 | 1488.8 | Trans-calamenene | 0.91 ± 0.01 |
| 15 | 28.64 | 1474 | 1497.2 | γ-Himachalene | 0.29 ± 0.01 |
| 16 | 29.07 | 1537 | 1507.5 | α-Calacorene | 1.34 ± 0.02 |
| Oxygenated Sesquiterpenoids | |||||
| 17 | 27.18 | 1763 | 1759.3 | Aristolone | 2.84 ± 0.04 |
| 18 | 29.94 | 1525 | 1528.4 | 9-Methoxycalamenene | 0.22 ± 0.01 |
| 19 | 30.04 | 1578 | 1531 | 1,5-Epoxysalvial-4(14)-ene | 1.48 ± 0.03 |
| 20 | 30.44 | 1576 | 1540.6 | Spathulenol | 2.49 ± 0.02 |
| 21 | 30.57 | 1580 | 1543.8 | (-)-Caryophyllene oxide | 5.36 ± 0.04 |
| 22 | 30.75 | 1530 | 1548.1 | Globulol | 2.21 ± 0.02 |
| 23 | 31.05 | 1504 | 1555.2 | Salvial-4(14)-en-1-one | 1.83 ± 0.02 |
| 24 | 31.46 | 1631 | 1595.2 | Aromadendrene oxide-(2) | 0.36 ± 0.01 |
| 25 | 31.69 | 1671 | 1667.9 | Calarene epoxide | 3.52 ± 0.03 |
| 26 | 32.04 | 1537 | 1579.4 | Isolongifolene, 7,8-dehydro-8a-hydroxy- | 5.06 ± 0.03 |
| 27 | 32.5 | 1608 | 1591.9 | (-)-Spathulenol | 7.54 ± 0.03 |
| 28 | 32.78 | 1582 | 1597.3 | Isoaromadendrene epoxide | 0.94 ± 0.01 |
| 29 | 33.07 | 1636 | 1604.5 | Tau-Muurolol | 1.76 ± 0.02 |
| 30 | 33.19 | 1763 | 1747.6 | Aristolene epoxide | 3.58 ± 0.03 |
| 31 | 33.49 | 1654 | 1615.4 | α-Eudesmol | 10.60 ± 0.03 |
| 32 | 33.92 | 1729 | 1726.1 | Murolan-3,9(11)-diene-10-peroxy | 0.37 ± 0.01 |
| 33 | 34.11 | 1548 | 1547.8 | Diepicedrene-1-oxide | 1.53 ± 0.02 |
| 34 | 34.85 | 1729 | 1739.7 | Ledene alcohol | 6.46 ± 0.03 |
| 35 | 42.24 | 1775 | 1756.3 | Furoscrobiculin B | 0.30 ± 0.01 |
| 36 | 48.67 | 2005 | 2045.9 | Isochiapin B | 0.53 ± 0.01 |
| 37 | 29.62 | 1653 | 1636.8 | (+) -γ- Costol | 2.80 ± 0.03 |
| Diterpenoids | |||||
| 38 | 39.7 | 2218 | 2215.6 | E-Phytol, acetate | 0.49 ± 0.01 |
| 39 | 47.78 | 2017 | 2018.4 | Kaur-16-ene, (8β,13β)- | 0.29 ± 0.01 |
| Oxygenated hydrocarbons | |||||
| 40 | 27.86 | 1512 | 1517.1 | Di-tert-Butylphenol | 0.85 ± 0.01 |
| 41 | 40.08 | 1840 | 1834.7 | 2-Pentadecanone, 6,10,14-trimethyl- | 1.23 ± 0.02 |
| 42 | 40.55 | 2566 | 2561.1 | Cis-13,16-Docasadienoic acid | 0.52 ± 0.01 |
| 43 | 41.15 | 2276 | 2278.9 | 11,14-Eicosadienoic acid, methyl ester | 0.73 ± 0.01 |
| Location | Egypt | Iran-Sistan | Iran-Lurestan | Pakistan | Brazil |
|---|---|---|---|---|---|
| Egypt | 1 | - | - | - | - |
| Iran-Sistan | −0.11 | 1 | - | - | - |
| Iran-Lurestan | −0.11 | 0.54 | 1 | - | - |
| Pakistan | −0.08 | 0.13 | 0.26 | 1 | - |
| Brazil | −0.04 | −0.01 | −0.01 | 0.02 | 1 |
| Treatment | Concentration (µL L−1) | LSD0.05 | |||
|---|---|---|---|---|---|
| 250 | 500 | 750 | 1000 | ||
| Germination | 70.43 c ± 1.78 | 82.47 b ± 2.09 | 90.77 a ± 2.30 | 97.34 a ± 2.46 | 7.08 |
| Root | 65.42 d ± 1.65 | 82.65 c ± 2.06 | 90.52 b ± 2.29 | 98.45 a ± 0.52 | 5.99 |
| Shoot | 48.56 d ± 1.23 | 73.42 c ± 1.86 | 84.52 b ± 2.14 | 93.56 c ± 2.15 | 6.34 |
| Concentration (µL L−1) | Scavenging (%) * |
|---|---|
| 500 | 58.45a ± 1.19 |
| 400 | 52.52b ± 0.79 |
| 300 | 50.20b ± 1.32 |
| 200 | 45.16c ± 1.56 |
| 100 | 38.56d ± 0.71 |
| LSD0.05 | 4.18 |
| IC50 µL L−1 | 321.93 |
| IC50 Ascorbic acid | 35.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gawad, A.A.; Elshamy, A.; El Gendy, A.E.-N.; Gaara, A.; Assaeed, A. Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium Strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules 2019, 24, 584. https://doi.org/10.3390/molecules24030584
El-Gawad AA, Elshamy A, El Gendy AE-N, Gaara A, Assaeed A. Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium Strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules. 2019; 24(3):584. https://doi.org/10.3390/molecules24030584
Chicago/Turabian StyleEl-Gawad, Ahmed Abd, Abdelsamed Elshamy, Abd El-Nasser El Gendy, Ahmed Gaara, and Abdulaziz Assaeed. 2019. "Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium Strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis" Molecules 24, no. 3: 584. https://doi.org/10.3390/molecules24030584
APA StyleEl-Gawad, A. A., Elshamy, A., El Gendy, A. E. -N., Gaara, A., & Assaeed, A. (2019). Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium Strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules, 24(3), 584. https://doi.org/10.3390/molecules24030584