Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Compounds
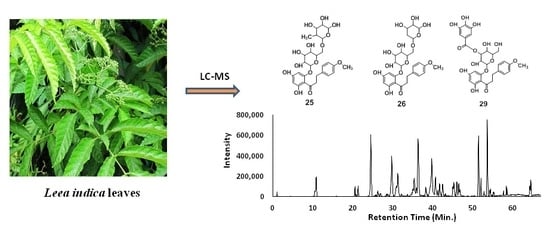
2.2. Identification of Dihydrochalcones by LC-ESI-MS/MS Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Extraction and Isolation
3.4. General Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ridsdale, C.E. Leeaceae. In Flora Malesiana, Series I–Spermatophyta Flowering Plants; Noordhoff International Publishing: Leiden, The Netherlands, 1976; Volume 7, pp. 755–782. [Google Scholar]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef]
- Siew, Y.Y.; Yew, H.C.; Neo, S.Y.; Seow, S.V.; Lew, S.M.; Lim, S.W.; Lim, C.S.E.S.; Ng, Y.C.; Seetoh, W.G.; Ali, A.; et al. Evaluation of anti-proliferative activity of medicinal plants used in Asian Traditional Medicine to treat cancer. J. Ethnopharmacol. 2019, 235, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bourdy, G.; Walter, A. Maternity and medicinal plants in Vanuatu I. The cycle of reproduction. J. Ethnopharmacol. 1992, 37, 179–196. [Google Scholar] [CrossRef]
- Singh, D.; Siew, Y.Y.; Yew, H.C.; Neo, S.Y.; Koh, H.L. Botany, phytochemistry and pharmacological activities of Leea species. In Medicinal Plants: Chemistry, Pharmacology, and Therapeutic Applications; Swamy, M.K., Patra, J.K., Rudramurthy, G.R., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2019; in press. [Google Scholar]
- Wiart, C.; Mogana, S.; Khalifah, S.; Mahan, M.; Ismail, S.; Buckle, M.; Narayana, A.K.; Sulaiman, M. Antimicrobial screening of plants used for traditional medicine in the state of Perak, Peninsular Malaysia. Fitoterapia 2004, 75, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Raihan, M.O.; Habib, M.R.; Brishti, A.; Rahman, M.M.; Saleheen, M.M.; Manna, M. Sedative and anxiolytic effects of the methanolic extract of Leea indica (Burm. f.) Merr. Leaf. Drug Discov. Ther. 2011, 5, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.H.; Kadir, H.A. Leea indica ethyl acetate fraction induces growth-inhibitory effect in various cancer cell lines and apoptosis in Ca Ski human cervical epidermoid carcinoma cells. Evid. Based Complement Alternat. Med. 2011. Available online: http://dx.doi.org/10.1155/2011/293060 (accessed on 18 January 2019).
- Reddy, N.S.; Navanesan, S.; Sinniah, S.K.; Wahab, N.A.; Sim, K.S. Phenolic content, antioxidant effect and cytotoxic activity of Leea indica leaves. BMC Complement Altern. Med. 2012, 12, 128. Available online: http://dx.doi.org./10.1186/1472-6882-12-128 (accessed on 18 January 2019). [CrossRef] [PubMed]
- Rahman, M.A.; Imran, T.B.; Islam, S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013, 20, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Avin, B.R.V.; Thirusangu, P.; Ramesh, C.K.; Vigneswarana, V.; Kumar, M.V.P.; Mahmood, R.; Prabhakar, B.T. Screening for the modulation of neovessel formation in non-tumorigenic and tumorigenic conditions using three different plants native to Western ghats of India. Biomed. Aging Pathol. 2014, 4, 343–348. [Google Scholar] [CrossRef]
- Mishra, G.; Khosa, R.L.; Singh, P.; Jha, K.K. Hepatoprotective activity of ethanolic extract of Leea indica (Burm. f.) Merr. (Leeaceae) stem bark against paracetamol induced liver toxicity in rats. Niger. J. Exp. Clin. Biosci. 2014, 2, 59–63. [Google Scholar] [CrossRef]
- Saha, K.; Shaari, K.; Lajis, N.H. Phytochemical study on Leea indica (Burm. F.) Merr. (Leeaceae). J. Bangladesh Chem. Soc. 2007, 20, 139–147. [Google Scholar]
- Srinivasan, G.V.; Ranjith, C.; Vijayan, K.K. Identification of chemical compounds from the leaves of Leea indica. Acta Pharm. 2008, 58, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.H.; Kadir, H.A.; Ling, S.K. Bioassay-guided isolation of cytotoxic cycloartane triterpenoid glycosides from the traditionally used medicinal plant Leea indica. Evid. Based Complement Alternat. Med. 2012. Available online: http://dx.doi.org/10.1155/2012/164689 (accessed on 18 January 2019).
- Liu, J.X.; Di, D.L.; Shi, Y.P. Diversity of chemical constituents from Saxifraga montana H. J. Chinese Chem. Soc. 2008, 55, 863–870. [Google Scholar] [CrossRef]
- Ekaprasada, M.T.; Nurdin, H.; Ibrahim, S.; Dachriyanus, H. Antioxidant activity of methyl gallate isolated from the leaves of Toona sureni. Indones. J. Chem. 2009, 9, 457–460. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Aderogba, M.A.; Ndhlala, A.R.; Rengasamy, K.R.R. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules 2013, 18, 12633–12644. [Google Scholar] [CrossRef]
- Silva, D.H.S.; Yoshida, M.; Kato, M.J. Flavonoids from Iryanthera sagotiana. Phytochemistry 1997, 46, 579–582. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, C.; Zang, Y.; Huang, Z.; Liu, G. The flavonoid composition of flavedo and juice from the pummel cultivar (Citrus grandis (L.) Osbeck) and the grapefruit cultivar (Citrus paradise). Food Chem. 2011, 129, 1530–1536. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, A.; Cahyanal, H.; Rahayu, D.U.C.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |





| Peak no. | RT (min) | Observed [M − H]− | Calculated [M − H]− | Error (ppm) | Molecular Formula | Fragment Ions (m/z) | Identified Compound |
|---|---|---|---|---|---|---|---|
| 1 | 10.9 | 169.0146 | 169.0142 | −2.2 | C7H6O5 | 125.0444 | Gallic acid |
| 2 | 15.9 | 305.0668 | 305.0667 | −0.4 | C15H14O7 | 261.0623, 219.0682, 179.0279, 167.0371, 165.0179, 151.1024 | Gallocatechin † |
| 3 | 20.6 | 327.0726 | 327.0722 | −1.4 | C14H16O9 | 312.0487, 234.0173, 207.0298, 206.0222, 192.0079 | Bergenin |
| 4 | 21.4 | 305.0668 | 305.0667 | −0.4 | C15H14O7 | 287.059, 261.076, 219.0694, 221.0473, 179.0362, 167.0387, 165.0199 | Epigallocatechin † |
| 5 | 24.5 | 183.0304 | 183.0299 | −2.7 | C8H8O5 | 169.0107 | Methyl gallate † |
| 6 | 26.3 | 913.1455 | 913.1469 | 1.6 | C44H34O22 | 761.1369, 743.1264, 609.1287, 591.1153, 573.1038, 447.0733, 423.0709, 285.0410, 169.0143 | Theasinensin A (isomer 1) † |
| 7 | 27.0 | 913.1471 | 913.1469 | −0.2 | C44H34O22 | 761.131, 743.1255, 609.1205, 591.1148, 573.1104, 447.0721, 423.0752, 285.0422, 169.0178 | Theasinensin A (isomer 2) † |
| 8 | 28.5 | 285.0399 | 285.0405 | 2.1 | C15H10O6 | 243.0291, 217.0528, 199.0420, 175.047 | Kaempferol |
| 9 | 28.8 | 289.0721 | 289.0718 | −1.0 | C15H14O6 | 221.0795, 203.0724, 175.0323 | Epicatechin |
| 10 | 29.8 | 457.0784 | 457.0776 | −1.6 | C22H18O11 | 305.0660, 261.0803, 219.0637, 169.0142 | Epigallocatechin-3-O-gallate † |
| 11 | 31.0 | 911.1315 | 911.1312 | −0.2 | C44H32O22 | 759.1258, 741.1135, 589.1027, 571.0861, 441.0556, 423.0727, 305.0618, 301.0453, 285.0431, 169.0135 | Theasinensin A quinone † |
| 12 | 32.2 | 897.1515 | 897.1520 | 0.5 | C44H34O21 | 745.1526, 727.1485, 575.1195, 557.1, 449.0938, 423.0693, 287.0576, 269.0482, 169.0127 | Theasinensin F † |
| 13 | 33.7 | 177.0191 | 177.0193 | 1.2 | C9H6O4 | 148.9428, 132.9003, 105.9031 | Esculetin † |
| 14 | 36.4 | 463.0886 | 463.0882 | −0.8 | C21H20O12 | 317.029, 316.0226, 287.0199, 271.0247, 179.0012, 135.8248 | Myricetin 3-O-rhamnoside (myricitrin) |
| 15 | 36.9 | 300.9989 | 300.9990 | 0.2 | C14H6O8 | 283.9927, 245.0151, 229.0091, 201.0309, 200.0171, 173.0194 | Ellagic acid † |
| 16 | 38.3 | 441.0831 | 441.0827 | −0.9 | C22H18O10 | 289.0701, 271.06, 245.9752, 169.0132 | Catechin gallate (isomer) † |
| 17 | 41.2 | 451.1254 | 451.1246 | −1.7 | C21H24O11 | 289.0724, 271.1548, 167.0353 | 3-Hydroxyphloridzin † |
| 18 | 41.7 | 447.0931 | 447.0933 | 0.4 | C21H20O11 | 301.0325, 300.0271, 255.0296, 179.0009 | Quercetin 3-O-rhamnoside (Quercitrin) |
| 19 | 43.2 | 417.0833 | 417.0827 | −0.6 | C20H18O10 | 284.0316, 257.0446, 255.0304, 227.0339 | Kaempferol 3-O-arabinoside † |
| 20 | 45.0 | 615.1001 | 615.0992 | −1.5 | C28H24O16 | 463.0903, 317.0319, 297.0616, 178.9989, 169.0188 | Myricetin-O-(O-galloyl)-3-rhamnopyranoside (isomer 1) † |
| 21 | 46.0 | 435.1299 | 435.1297 | −0.5 | C21H24O10 | 273.0758, 167.0349 | Phloridzin |
| 22 | 46.5 | 615.0988 | 615.0992 | 0.6 | C28H24O16 | 463.0817, 317.0332, 297.0677, 178.9976, 169.011 | Myricetin-O-(O-galloyl)-3-rhamnopyranoside (isomer 2) † |
| 23 | 46.8 | 315.0146 | 315.0146 | 0.1 | C15H8O8 | 299.9902, 270.9912, 243.9987, 151.0037 | Methyl-O-ellagic acid † |
| 24 | 50.4 | 599.1048 | 599.1042 | −1.0 | C28H24O15 | 447.0893, 301.0369, 169.0125, 151.8637 | Quercitrin 2″-O-gallate † |
| 25 | 51.4 | 595.2031 | 595.2032 | 0.2 | C28H36O14 | 433.1347, 329.1078, 308.2508, 287.0929, 167.0376 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-rutinoside † |
| 26 | 52.1 | 581.1889 | 581.1876 | −0.5 | C27H34O14 | 419.1210, 329.102, 311.0951, 293.0907, 287.0926, 273.0953, 243.1026, 167.0355 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-glucosylpentoside † |
| 27 | 53.6 | 449.1452 | 449.1453 | 0.4 | C22H26O10 | 329.1080, 287.0921, 273.0744, 272.0683, 243.1032, 181.017, 167.0298, 166.0275, 151.0067 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-β-d-glucopyranoside † |
| 28 | 54.5 | 327.2171 | 327.2177 | 1.8 | C18H32O5 | 309.2164, 298.9867, 291.1998, 239.1283, 229.1447, 211.1327, 183.0131, 171.103 | 9,12,13-Trihydroxy octadecadienoic acid † |
| 29 | 55.0 | 601.1595 | 601.1563 | −5.3 | C29H30O14 | 439.0901, 329.1098, 313.0559, 287.0914, 271.0502, 243.1106, 211.0199, 169.0167 | 4′,6′-Dihydroxy-4-methoxy dihydrochalcone 2′-O-(3″-O-galloyl)-β-d-glucopyranoside † |
| 30 | 57.7 | 221.1186 | 221.1183 | −1.4 | C13H18O3 | 149.0978 | Dehydrovomifoliol † |
| 31 | 58.6 | 287.0926 | 287.0925 | −0.2 | C16H16O5 | 243.1034, 167.037, 151.0043 | 2′,4′,6′-Trihydroxy-4-methoxy dihydrochalcone (3-Methylphloretin) † |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Siew, Y.-Y.; Chong, T.-I.; Yew, H.-C.; Ho, S.S.-W.; Lim, C.S.E.-S.; Tan, W.-X.; Neo, S.-Y.; Koh, H.-L. Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry. Molecules 2019, 24, 714. https://doi.org/10.3390/molecules24040714
Singh D, Siew Y-Y, Chong T-I, Yew H-C, Ho SS-W, Lim CSE-S, Tan W-X, Neo S-Y, Koh H-L. Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry. Molecules. 2019; 24(4):714. https://doi.org/10.3390/molecules24040714
Chicago/Turabian StyleSingh, Deepika, Yin-Yin Siew, Teck-Ian Chong, Hui-Chuing Yew, Samuel Shan-Wei Ho, Claire Sophie En-Shen Lim, Wei-Xun Tan, Soek-Ying Neo, and Hwee-Ling Koh. 2019. "Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry" Molecules 24, no. 4: 714. https://doi.org/10.3390/molecules24040714
APA StyleSingh, D., Siew, Y. -Y., Chong, T. -I., Yew, H. -C., Ho, S. S. -W., Lim, C. S. E. -S., Tan, W. -X., Neo, S. -Y., & Koh, H. -L. (2019). Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry. Molecules, 24(4), 714. https://doi.org/10.3390/molecules24040714