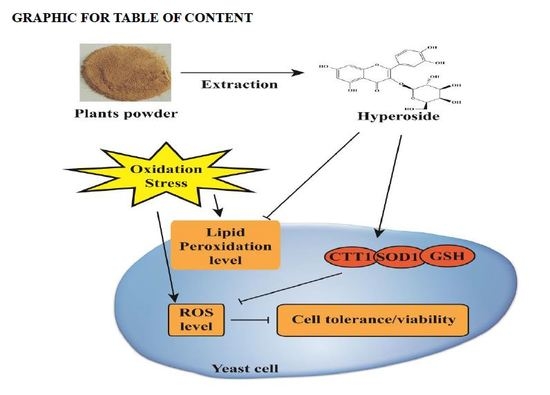
Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity Assay of Hyperoside
2.2. Hyperoside Increases Oxidative Stress Tolerance in S. cerevisiae
2.3. Hyperoside Attenuates the Level of Intracellular Oxidation
2.4. Hyperoside Attenuates S. cerevisiae Cell Membrane Lipid Peroxidation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. S. cerevisiae Strains, Media, and Growth Conditions
3.3. Cytotoxicity Assay of Hyperoside
3.4. Tolerance Assay
3.5. Cell Growth Assay
3.6. Determination of Cell Membrane Lipid Peroxidation
3.7. Determination of Intracellular Oxidation
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SODs | superoxide dismutases |
| GPXs | glutathione peroxidases |
| CTT1 | cytosolic catalaseT1 |
| Prxs | peroxiredoxins |
| GSH | glutathione |
| ROS | reactive oxygen species |
| S.cerevisiae | saccharomyces cerevisiae |
| GTT1 | glutathione transferase1 |
| GTT2 | glutathione transferase2 |
| YPD | yeast extract peptone dextrose |
| TCA | trichloroacetic acid |
| TBA | thiobarbituric acid |
| MDA | malondialdehyde |
| H2DCF-DA | 2′7′-dichlorofluorescein diacetate |
| WT | wild type |
References
- Meng, D.; Zhang, P.; Zhang, L.L.; Wang, H.; Ho, C.T.; Li, S.M.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Li, S.M.; Ho, C.T.; Zhao, H. Antioxidant activity evaluation of dietary phytochemicals using Saccharomyces cerevisiae as a model. J. Funct. Foods 2017, 38, 36–44. [Google Scholar] [CrossRef]
- Wang, L.W.; Wang, J.H.; Fang, L.Y.; Zheng, Z.L.; Zhi, D.X.; Wang, S.Y.; Li, S.M.; Ho, C.T.; Zhao, H. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. Biomed. Res. Int. 2014, 2014, 453972. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, S.M.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Pan, M.H.; Lo, C.Y.; Zhao, H.; Li, S.M.; Ho, C.T.; Yang, G.L. Anti-fibrotic activity of polyphenol-enriched sugarcane extract in rats via inhibition of p38 and JNK phosphorylation. Food Funct. 2018, 9, 951–958. [Google Scholar]
- Su, D.X.; Li, N.; Chen, M.; Yang, Y.; He, S.; Wang, Y.; Wu, Q.H.; Li, L.; Yang, H.L.; Zeng, Q.Z. Effects of in vitro digestion on the composition of flavonoids and antioxidant activities of the lotus leaf at different growth stages. Int. J. Food Sci. Technol. 2018, 53, 1631–1639. [Google Scholar] [CrossRef]
- Shukor, N.A.A.; Ablat, A.; Muhamad, N.A.; Mohamad, J. In vitro antioxidant and in vivo xanthine oxidase inhibitory activities of Pandanus amaryllifolius in potassium oxonate-induced hyperuricemic rats. Int. J. Food Sci. Technol. 2018, 53, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Quan, W.; Tao, Y.D.; Lu, M.; Yuan, B.; Chen, J.; Zeng, M.M.; Qin, F.; Guo, F.X.; He, Z.Y. Stability of the phenolic compounds and antioxidant capacity of five fruit (apple, orange, grape, pomelo and kiwi) juices during in vitro simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2018, 53, 1131–1139. [Google Scholar] [CrossRef]
- Elijah, A.A.; Daniel, M.C.; Porfirio, M.; Mercedes, S.; Helios, E.; Marıa, E.M.; Azalia, A.N.; Ivan, C.; Myrna, B. Comparative study of antioxidant and antibacterial properties of the edible mushrooms Pleurotus levis, P. ostreatus, P. pulmonarius and P. tuber-regium. Int. J. Food Sci. Technol. 2018, 53, 1316–1330. [Google Scholar]
- Ahmad, H.S.M.; Ismail, A.; Kassim, N.K.; Hamid, M.; Mat, A.M.S. Underutilised fruits: A review of phytochemistry and biological properties. J. Food Bioact. 2018, 1, 28. [Google Scholar]
- Mukherjee, S.; Das, S.K.; Vasudevan, D.M. Protective role of extracts of grape skin and grape flesh on ethanol-induced oxidative stress, inflammation and histological alterations in rat brain. Arch. Physiol. Biochem. 2015, 121, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M.; Xu, Y.F.; Fang, Y.; Che, J.P.; Wang, G.C.; Zheng, J.H. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncol. Rep. 2014, 31, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.Y.; Cai, Y.Q.; Wang, X.F.; Wang, L.L.; Li, P.; Wang, G.Y.; Chen, J.H. The Cytoprotective Effect of Hyperoside against Oxidative Stress Is Mediated by the Nrf2-ARE Signaling Pathway through GSK-3beta Inactivation. PLoS ONE 2015, 10, e0145183. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Liu, M.; Li, W.; Che, J.P.; Wang, G.C.; Zheng, J.H. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA21. Mol. Med. Rep. 2015, 11, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Maimoona, A.; Abbas, G.; Naeem, I.; Shahzad, M. Pharmacological screening of Hypericum androsaemum extracts for antioxidant, anti-lipid peroxidation, antiglycation and cytotoxicity activity. Pak. J. Pharm. Sci. 2016, 29, 397–405. [Google Scholar] [PubMed]
- Wang, L.W.; Yue, Z.W.; Guo, M.Z.; Fang, L.Y.; Bai, L.; Li, X.Y.; Tao, Y.Q.; Wang, S.Y.; Liu, Q.; Zhi, D.X.; et al. Dietary Flavonoid Hyperoside Induces Apoptosis of Activated Human LX-2 Hepatic Stellate Cell by Suppressing Canonical NF-kappaB Signaling. BioMed. Res. Int. 2016, 2016, 1068528. [Google Scholar] [PubMed]
- Wen, L.; Lin, Y.L.; Lv, R.M.; Yan, H.J.; Yu, J.Q.; Zhao, H.Q.; Wang, X.; Wang, D.J. An Efficient Method for the Preparative Isolation and Purification of Flavonoids from Leaves of Crataegus pinnatifida by HSCCC and Pre-HPLC. Molecules 2017, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Wang, Y.W.; Li, L.; Kong, R.; Pan, S.H.; Ji, L.; Liu, H.; Chen, H.; Sun, B. Hyperoside induces apoptosis and inhibits growth in pancreatic cancer via Bcl-2 family and NF-kappaB signaling pathway both in vitro and in vivo. Tumor. Biol. 2016, 37, 7345–7355. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Zhang, R.; Ko, D.O.; Wang, Z.H.; You, H.J.; Kim, H.S.; Kim, J.S.; Kang, S.S.; Hyun, J.W. Hyperoside prevents oxidative damage induced by hydrogen peroxide in lung fibroblast cells via an antioxidant effect. Bba Biomembranes 2008, 1780, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M.; Jakstas, V.; Majiene, D.; Baniene, R.; Kuršvietiene, L.; Masteikova, R.; Savickas, A.; Toleikis, A.; Trumbeckaite, S. The effect of Leonurus cardiaca herb extract and some of its flavonoids on mitochondrial oxidative phosphorylation in the heart. Planta Med. 2014, 80, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, M.M.; Shabanah, O.A.A.; Harbi, N.A.O.; Harbi, M.M.A.; Rejaie, S.S.A.; Alsurayea, S.M.; Ahmed, M.M.S. Association between paraoxonases gene expression and oxidative stress in hepatotoxicity induced by CCl4. Oxid. Med. Cell Longev. 2014, 2014, 893212. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ghassemian, M.; Komives, E.A.; Russell, P. Cadmium-induced proteome remodeling regulated by Spc1/Sty1 and Zip1 in fission yeast. Toxicol. Sci. 2012, 129, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of a Flavonoid-Rich Extract of Hypericum perforatum L. in Vitro. J. Agri. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debattisti, V.; Gerencser, A.A.; Saotome, M.; Das, S.; Hajnoczky, G. ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak. Cell Rep. 2017, 21, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Rezaee, M.; Sahebkar, A. Cadmium-induced toxicity is rescued by curcumin: A review. Biofactors 2017, 43, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pant, A.; Trivedi, S.; Pandey, R. Curcumin and beta-caryophellene attenuate cadmium quantum dots induced oxidative stress and lethality in Caenorhabditis elegans model system. Environ. Toxicol. Phar. 2016, 42, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; Marchi, E.D.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-ros crosstalk in the control of cell death and aging. J. Recept. Signal Transduct. Res. 2012, 2012, 329635. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Sytykiewicz, H.; Durak, R.; Sobkowiak, B.B. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol. Bioch. 2017, 118, 529–540. [Google Scholar] [CrossRef]
- Sandoval-Acuna, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ying, M.D.; Wu, Y.P.; Zhou, Z.H.; Ye, Z.M.; Li, H.; Lin, D.S. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef] [PubMed]
- Asimi, O.A.; Sahu, N.P.; Pal, A.K. Antioxidant capacity of crude water and ethylacetate extracts of some Indian spices and their antimicrobial activity against Vibrio vulnificus and Micrococcus luteus. J. Med. Plants Res. 2013, 7, 1907–1915. [Google Scholar]
- Jennifer Spencer, T.G.P.; Katherine, A.S.; Greetham, D. Tolerance of pentose utilising yeast to hydrogen peroxide-induced oxidative stress. BMC Res. Notes 2014, 7, 1–12. [Google Scholar]
- Alan, T.; Williams, M.D.; Raymond, F.; Burk, M.D. Carbon Tetrachloride Hepatotoxicity: An Example of Free Radical-Mediated Injury. Seminars Liver Dis. 1990, 10, 279–284. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Clarendon Press: Oxford, UK, 1999. [Google Scholar]
- Amamou, F.; Nemmiche, S.; Nemmiche, R.K.; Didi, A.; Yazit, S.M.; Sari, D.C. Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem. Toxicol. 2015, 78, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Shagirtha, K. Hesperetin protects against oxidative stress related hepatic dysfunction by cadmium in rats. Exp. Toxicol. Pathol. 2012, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.A.; Bayomy, M.F.; El-Morsy, A.M. Rosemary extract ameliorates cadmium-induced histological changes and oxidative damage in the liver of albino rat. J. Basic Appl. Zool. 2015, 71, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. BBA Biomembranes 2006, 1763, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Gene 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Perrone, G.G.; Kristiansson, E.; Traini, M.; Ye, T.; Dawes, I.W.; Nerman, O.; Tamás, M.J. Genetic basis of arsenite and cadmium tolerance in Saccharomyces cerevisiae. BMC Genomics 2009, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Venancio, V.P.; Cipriano, P.A.; Kim, H.; Antunes, L.M.G.; Talcott, S.T.; Mertens-Talcott, S.U. Cocoplum (Chrysobalanus icaco L.) anthocyanins exert anti-inflammatory activity in human colon cancer and non-malignant colon cells. Food Funct. 2017, 8, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, C.; Vitale, M.; Griffo, E.; Giacco, A.; Natale, C.D.; Cocozza, S.; et al. Polyphenol-rich diets improve glucose metabolism in people at high cardiometabolic risk: a controlled randomised intervention trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Said, J.W.; Huang, M.; Grogan, T.; Elashoff, D.; Carpenter, C.L.; Heber, D.; Aronson, W.J. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate 2015, 75, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzoska, M.M. Review of polyphenol-rich products as potential protective and therapeutic factors against cadmium hepatotoxicity. J. App. Toxicol. 2019, 39, 117–145. [Google Scholar] [CrossRef]
- Jancic, S.A.; Stosic, B.Z. Cadmium effects on the thyroid gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. Lipid Peroxidation 2012. [Google Scholar] [Green Version]
- Knockaert, L.; Berson, A.; Ribault, C.; Prost, P.E.; Fautrel, A.; Pajaud, J.; Lepage, S.; Lucas-Clerc, C.; Begue´, J.M.; Fromenty, B.; et al. Carbon tetrachloride-mediated lipid peroxidation induces early mitochondrial alterations in mouse liver. Lab. Invest. 2012, 92, 396–410. [Google Scholar] [CrossRef]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radical. Bio. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiba, A.S.; Abbiyesuku, F.M.; Niran-atiba, T.A.; Oparinde, D.P.; Ajose, O.A.; Akindele, R.A. Free Radical Attack on Membrane Lipid and Antioxidant Vitamins in the Course of Pre-Eclamptic Pregnancy. Ethiop. J. Health Dev. 2014, 24, 35. [Google Scholar] [CrossRef] [Green Version]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; Sousa, D.P. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxid. Med. Cell Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.N.; Mannarino, S.C.; Silva, C.G.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C.A. Oxidative stress response in eukaryotes: effect of glutathione, superoxide dismutase and catalase on adaptation to peroxide and menadione stresses in Saccharomyces cerevisiae. Redox Rep. 2007, 12, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Mauro Braga Franca, A.D.P.; Eleutherio, E.C.A. The role of cytoplasmic catalase in dehydration tolerance of Saccharomyces cerevisiae. Cell Stress Chaper. 2015, 10, 3. [Google Scholar]
- Kaosaar, S.; Kahru, A.; Mantecca, P.; Kasemets, K. Profiling of the toxicity mechanisms of coated and uncoated silver nanoparticles to yeast Saccharomyces cerevisiae BY4741 using a set of its 9 single-gene deletion mutants defective in oxidative stress response, cell wall or membrane integrity and endocytosis. TIV 2016, 35, 149–162. [Google Scholar]
- Pereira, M.D.; Herdeiro, R.S.; Fernandes, P.N.; Eleutherio, E.C.A.; Panek, A.D. Targets of oxidative stress in yeast sod mutants. BBA Gen. Subjects 2003, 1620, 245–251. [Google Scholar] [CrossRef]
- Silva, C.G.; Herdeiro, R.S.; Mathias, J.C.; Panek, A.D.; Silveira, C.S.; Rodrigues, V.P.; Renno, M.N.; Falc˜ao, D.Q.; Cerqueira, D.M.; Minto, A.B.M.; et al. Evaluation of antioxidant activity of Brazilian plants. Pharmacol. Res. 2005, 52, 229–233. [Google Scholar] [CrossRef]
- Bompiani, K.M.; Tsai, C.Y.; Achatz, F.P.; Liebig, J.K.; Howell, S.B. Copper transporters and chaperones CTR1, CTR2, ATOX1, and CCS as determinants of cisplatin sensitivity. Metallomics 2016, 8, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Baron, J.A.; Chen, J.S.; Culotta, V.C. Cu/Zn superoxide dismutase and the proton ATPase Pma1p of Saccharomyces cerevisiae. Biochem. Hem. Bio. Res. Co. 2015, 462, 251–260. [Google Scholar] [CrossRef]
- Dani, C.; Bonatto, D.; Salvador, M.; Pereira, M.D.; Henriques, J.A.P.; Eleutherio, E. Antioxidant Protection of Resveratrol and Catechin in Saccharomyces cerevisiae. J. Agri. Food Chem. 2008, 56, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Meng, D.; Zhang, P.; Wang, X.X.; Du, G.; Brennan, C.; Li, S.M.; Ho, C.H.; Zhao, H. Antioxidant Protection of Nobiletin, 5-Demethylnobiletin, Tangeretin, and 5-Demethyltangeretin from Citrus Peel in Saccharomyces cerevisiae. J. Agri. Food Chem. 2018, 66, 3155–3160. [Google Scholar] [CrossRef] [PubMed]



| Stress | Treatment | WT | sod1Δ | ctt1Δ | gsh1Δ |
|---|---|---|---|---|---|
| CCl4 | without hyperoside | 4.77 ± 0.53 p a q | 3.35 ± 0.43b | 2.49 ± 0.47c | 2.41 ± 0.31c |
| with hyperoside | 1.62 ± 0.40d | 1.69 ± 0.23d | 2.36 ± 0.22c | 1.45 ± 0.16d | |
| H2O2 | without hyperoside | 14.23 ± 0.31a | 6.99 ± 0.20b | 5.84 ± 0.39c | 8.87 ± 0.52b |
| with hyperoside | 4.39 ± 0.33c | 1.87 ± 0.45d | 5.86 ± 0.14c | 1.24 ± 0.17d | |
| CdSO4 | without hyperoside | 1.91 ± 0.23a | 1.84 ± 0.56a | 2.00 ± 0.15a | 1.60 ± 0.30a |
| with hyperoside | 0.70 ± 0.57d | 1.72 ± 0.24a | 1.13 ± 0.17c | 1.44 ± 0.29a |
| Stress | Treatment | WT | sod1Δ | ctt1Δ | gsh1Δ |
|---|---|---|---|---|---|
| CCl4 | without hyperoside | 1.9 ± 0.3paq | 1.5 ± 0.2a | 1.7 ± 0.2a | 1.6 ± 0.3a |
| with hyperoside | 0.7 ± 0.2c | 0.8 ± 0.3b | 0.6 ± 0.3c | 0.5 ± 0.2c | |
| H2O2 | without hyperoside | 2.3 ± 0.3a | 2.1 ± 0.2a | 2.5 ± 0.2a | 2.4 ± 0.3a |
| with hyperoside | 1.0 ± 0.2c | 1.2 ± 0.3b | 1.3 ± 0.1b | 1.2 ± 0.2b | |
| CdSO4 | without hyperoside | 1.5 ± 0.2a | 1.2 ± 0.1a | 1.3 ± 0.2a | 1.4 ± 0.2a |
| with hyperoside | 1.1 ± 0.3b | 0.6 ± 0.2c | 0.8 ± 0.2c | 0.7 ± 0.1c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H.; et al. Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model. Molecules 2019, 24, 788. https://doi.org/10.3390/molecules24040788
Gao Y, Fang L, Wang X, Lan R, Wang M, Du G, Guan W, Liu J, Brennan M, Guo H, et al. Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model. Molecules. 2019; 24(4):788. https://doi.org/10.3390/molecules24040788
Chicago/Turabian StyleGao, Yuting, Lianying Fang, Xiangxing Wang, Ruoni Lan, Meiyan Wang, Gang Du, Wenqiang Guan, Jianfu Liu, Margaret Brennan, Hongxing Guo, and et al. 2019. "Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model" Molecules 24, no. 4: 788. https://doi.org/10.3390/molecules24040788
APA StyleGao, Y., Fang, L., Wang, X., Lan, R., Wang, M., Du, G., Guan, W., Liu, J., Brennan, M., Guo, H., Brennan, C., & Zhao, H. (2019). Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model. Molecules, 24(4), 788. https://doi.org/10.3390/molecules24040788