Single Molecule Fluorescence Spectroscopy of PSI Trimers from Arthrospira platensis: A Computational Approach
Abstract
:1. Introduction
2. PSI Structure
3. Comparison of Different PSIs
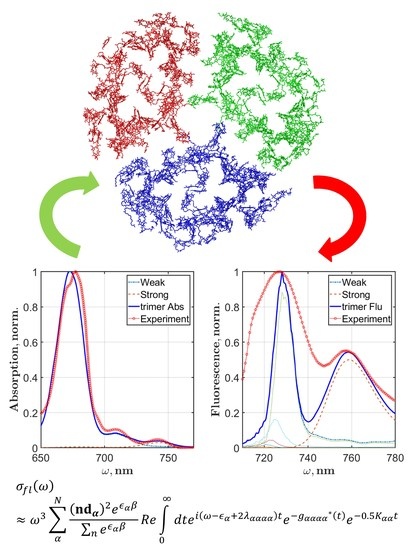
4. Arthrospira platensis PSI Trimer
5. Results
5.1. Chl Monomer
5.2. Linear Spectra of PSI
5.3. Fluorescence Spectra of PSI Trimer
6. Discussion
7. Computational Procedures
7.1. Chl Absorption Modeling
7.2. Theory of Optical Response for PSI
7.3. Computational Parameters for PSI Monomer and Trimer Models
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Mode | Mode | Mode | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | 0.5 | 18 | 752 | 0.0067 | 35 | 1304 | 0.006 |
| 2 | 85 | 0.06 | 19 | 795 | 0.005 | 36 | 1322 | 0.030 |
| 3 | 138 | 0.085 | 20 | 916 | 0.026 | 37 | 1338 | 0.004 |
| 4 | 213 | 0.001 | 21 | 986 | 0.010 | 38 | 1354 | 0.006 |
| 5 | 260 | 0.027 | 22 | 995 | 0.001 | 39 | 1382 | 0.007 |
| 6 | 298 | 0.0002 | 23 | 1052 | 0.012 | 40 | 1439 | 0.007 |
| 7 | 342 | 0.060 | 24 | 1069 | 0.0053 | 41 | 1487 | 0.008 |
| 8 | 388 | 0.0001 | 25 | 1110 | 0.011 | 42 | 1524 | 0.006 |
| 9 | 425 | 0.014 | 26 | 1143 | 0.042 | 43 | 1537 | 0.022 |
| 10 | 518 | 0.0003 | 27 | 1181 | 0.018 | 44 | 1553 | 0.009 |
| 11 | 546 | 0.003 | 28 | 1190 | 0.0011 | 45 | 1573 | 0.005 |
| 12 | 573 | 0.0004 | 29 | 1208 | 0.019 | 46 | 1580 | 0.005 |
| 13 | 585 | 0.003 | 30 | 1216 | 0.0045 | 47 | 1612 | 0.004 |
| 14 | 604 | 0.0017 | 31 | 1235 | 0.007 | 48 | 1645 | 0.004 |
| 15 | 700 | 0.002 | 32 | 1252 | 0.006 | 49 | 1673 | 0.001 |
| 16 | 722 | 0.0019 | 33 | 1260 | 0.006 | |||
| 17 | 742 | 0.039 | 34 | 1286 | 0.005 |
| Chl | , cm−1 | Chl | , cm−1 | Chl | cm−1 | Chl | cm−1 |
|---|---|---|---|---|---|---|---|
| P700A | 15011 | A19 | 14967 | B03 | 15019 | B27 | 14951 |
| P700B | 15011 | A20 | 15046 | B04 | 14985 | B28 | 15011 |
| AccA | 14946 | A21 | 15044 | B05 | 14973 | B29 | 15015 |
| AccB | 15113 | A22 | 14924 | B06 | 14975 | B30 | 14931 |
| A0A | 15014 | A23 | 15016 | B07 | 15144 | B31 | 14975 |
| A0B | 14932 | A24 | 14953 | B08 | 14928 | B32 | 14924 |
| A01 | 14921 | A25 | 15004 | B09 | 14997 | B33 | 14920 |
| A02 | 14990 | A26 | 14940 | B10 | 14962 | B34 | 14985 |
| A03 | 15141 | A27 | 14975 | B11 | 14980 | B35 | 14929 |
| A04 | 14968 | A28 | 14444 | B12 | 14980 | B36 | 14963 |
| A05 | 15111 | A29 | 15003 | B13 | 15012 | B37 | 14971 |
| A06 | 15139 | A30 | 14981 | B14 | 15059 | B38 | 14975 |
| A07 | 15015 | A31 | 14924 | B15 | 14935 | B39 | 14470 |
| A08 | 14984 | A32 | 14992 | B16 | 14989 | J01 | 14949 |
| A09 | 14984 | A33 | 14935 | B17 | 14982 | J02 | 14971 |
| A10 | 14975 | A34 | 15047 | B18 | 15080 | J03 | 14925 |
| A11 | 14985 | A35 | 14966 | B19 | 15145 | K01 | 14964 |
| A12 | 15004 | A36 | 15005 | B20 | 15039 | K02 | 15013 |
| A13 | 15036 | A37 | 14999 | B21 | 14973 | L01 | 14934 |
| A14 | 15002 | A38 | 14962 | B22 | 14950 | L02 | 14926 |
| A15 | 14997 | A39 | 15012 | B23 | 15096 | L03 | 14925 |
| A16 | 15019 | A40 | 14406 | B24 | 15063 | M41 | 14962 |
| A17 | 14999 | B01 | 14995 | B25 | 15097 | X42 | 14964 |
| A18 | 14966 | B02 | 14952 | B26 | 14426 | PL43 | 14941 |
References
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2014; p. 312. [Google Scholar]
- Pishchalnikov, R.Y.; Razjivin, A.P. From localized excited states to excitons: Changing of conceptions of primary photosynthetic processes in the twentieth century. Biochemistry 2014, 79, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Shevela, D.; Pishchainikov, R.Y.; Eichacker, L.A. Govindjee, Oxygenic Photosynthesis in Cyanobacteria. In Stress Biology of Cyanobacteria: Molecular Mechanism to Cellular Responses; CRC Press: Boca Raton, FL, USA, 2013; pp. 3–40. [Google Scholar]
- van Grondelle, R.; van Gorkom, H. The birth of the photosynthetic reaction center: The story of Lou Duysens. Photosynth. Res. 2014, 120, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pishchalnikov, R.Y.; Pershin, S.M.; Bunkin, A.F. H2O and D2O spin-isomers as a mediator of the electron transfer in the reaction center of purple bacteria. Phys. Wave Phenom. 2012, 20, 184–192. [Google Scholar] [CrossRef]
- Rakhimberdieva, M.G.; Boichenko, V.A.; Karapetyan, N.V.; Stadnichuk, I.N. Interaction of phycobilisomes with photosystem II dimers and photosystem I monomers and trimers in the cyanobacterium Spirulina platensis. Biochemistry 2001, 40, 15780–15788. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. Regulatory network of proton motive force: Contribution of cyclic electron transport around photosystem I. Photosynth. Res. 2016, 129, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, S.J.; Zhang, S.B.; Zhang, J.L.; Cao, K.F. Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 2012, 235, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kubota, H.; Wada, H.; Narikawa, R.; Ikeuchi, M. Novel Supercomplex Organization of Photosystem I in Anabaena and Cyanophora paradoxa. Plant Cell Physiol. 2011, 52, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauss, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 angstrom resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Malavath, T.; Caspy, I.; Netzer-El, S.Y.; Klaiman, D.; Nelson, N. Structure and function of wild-type and subunit-depleted photosystem I in Synechocystis. BBA-Bioenergetics 2018, 1859, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, N.V.; Bolychevtseva, Y.V.; Yurina, N.P.; Terekhova, I.V.; Shubin, V.V.; Brecht, M. Long-wavelength chlorophylls in photosystem I of cyanobacteria: Origin, localization, and functions. Biochemistry 2014, 79, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Brecht, M.; Hussels, M.; Schlodder, E.; Karapetyan, N.V. Red antenna states of Photosystem I trimers from Arthrospira platensis revealed by single-molecule spectroscopy. BBA-Bioenergetics 2012, 1817, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karapetyan, N.V.; Holzwarth, A.R.; Rogner, M. The photosystem I trimer of cyanobacteria: Molecular organization, excitation dynamics and physiological significance. Febs Lett. 1999, 460, 395–400. [Google Scholar] [CrossRef]
- Palsson, L.O.; Flemming, C.; Gobets, B.; van Grondelle, R.; Dekker, J.P.; Schlodder, E. Energy transfer and charge separation in photosystem I: P700 oxidation upon selective excitation of the long-wavelength antenna chlorophylls of Synechococcus elongatus. Biophys. J. 1998, 74, 2611–2622. [Google Scholar] [CrossRef]
- Bruggemann, B.; Sznee, K.; Novoderezhkin, V.; van Grondelle, R.; May, V. From structure to dynamics: Modeling exciton dynamics in the photosynthetic antenna PS1. J. Phys. Chem. B 2004, 108, 13536–13546. [Google Scholar] [CrossRef]
- Damjanovic, A.; Vaswani, H.M.; Fromme, P.; Fleming, G.R. Chlorophyll excitations in photosystem I of Synechococcus elongatus. J. Phys. Chem. B 2002, 106, 10251–10262. [Google Scholar] [CrossRef]
- Byrdin, M.; Jordan, P.; Krauss, N.; Fromme, P.; Stehlik, D.; Schlodder, E. Light harvesting in photosystem I: Modeling based on the 2.5-angstrom structure of photosystem I from Synechococcus elongatus. Biophys. J. 2002, 83, 433–457. [Google Scholar] [CrossRef]
- Schlodder, E.; Cetin, M.; Byrdin, M.; Terekhova, I.V.; Karapetyan, N.V. P700(+)- and P700-induced quenching of the fluorescence at 760 nm in trimeric Photosystem I complexes from the cyanobactenium Arthrospira platensis. BBA-Bioenergetics 2005, 1706, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Shubin, V.V.; Tsuprun, V.L.; Bezsmertnaya, I.N.; Karapetyan, N.V. Trimeric forms of the photosystem-i reaction-center complex pre-exist in the membranes of the cyanobacterium spirulina-platensis. Febs Lett. 1993, 334, 79–82. [Google Scholar] [CrossRef]
- Schlodder, E.; Hussels, M.; Cetin, M.; Karapetyan, N.V.; Brecht, M. Fluorescence of the various red antenna states in photosystem I complexes from cyanobacteria is affected differently by the redox state of P700. BBA-Bioenergetics 2011, 1807, 1423–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kompanets, V.; Shubin, V.; Terekhova, I.; Kotova, E.; Kozlovsky, V.; Novoderezhkin, V.; Chekalin, S.; Karapetyan, N.; Razjivin, A. Red chlorophyll excitation dynamics in Arthrospira platensis photosystem I trimeric complexes as studied by femtosecond transient absorption spectroscopy. Febs Lett. 2014, 588, 3441–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukamel, S. Principles of Nonlinear Optical Spectroscopy; Oxford University Press: New York, NY, USA, 1995; Volume 6, p. 543. [Google Scholar]
- Lu, H.P.; Xie, X.S. Single-molecule spectral fluctuations at room temperature. Nature 1997, 385, 143–146. [Google Scholar] [CrossRef]
- Jung, Y.J.; Barkai, E.; Silbey, R.J. Current status of single-molecule spectroscopy: Theoretical aspects. J. Chem. Phys. 2002, 117, 10980–10995. [Google Scholar] [CrossRef]
- Barkai, E.; Jung, Y.J.; Silbey, R. Theory of single-molecule spectroscopy: Beyond the ensemble average. Annual Rev. Phys. Chem. 2004, 55, 457–507. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.M.; Maly, P.; Kruger, T.P.J.; van Grondelle, R. From isolated light-harvesting complexes to the thylakoid membrane: A single-molecule perspective. Nanophotonics 2018, 7, 81–92. [Google Scholar] [CrossRef]
- Schlau-Cohen, G.S.; Bockenhauer, S.; Wang, Q.; Moerner, W.E. Single-molecule spectroscopy of photosynthetic proteins in solution: Exploration of structure-function relationships. Chem. Sci. 2014, 5, 2933–2939. [Google Scholar] [CrossRef]
- Gall, A.; Ilioaia, C.; Kruger, T.P.J.; Novoderezhkin, V.I.; Robert, B.; van Grondelle, R. Conformational Switching in a Light-Harvesting Protein as Followed by Single-Molecule Spectroscopy. Biophys. J. 2015, 108, 2713–2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkauskas, D.; Novoderezkhin, V.; Cogdell, R.J.; van Grondelle, R. Fluorescence spectral fluctuations of single LH2 complexes from Rhodopseudomonas acidophila strain 10050. Biochemistry 2004, 43, 4431–4438. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.P.J.; Novoderezhkin, V.I.; Ilioaia, C.; van Grondelle, R. Fluorescence Spectral Dynamics of Single LHCII Trimers. Biophys. J. 2010, 98, 3093–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skandary, S.; Konrad, A.; Hussels, M.; Meixner, A.J.; Brecht, M. Orientations between Red Antenna States of Photosystem I Monomers from Thermosynechococcus elongatus Revealed by Single-Molecule Spectroscopy. J. Phys. Chem. B 2015, 119, 13888–13896. [Google Scholar] [CrossRef] [PubMed]
- Skandary, S.; Konrad, A.; Hussels, M.; Meixner, A.J.; Brecht, M. Polarization Dependent Single-Molecule Spectroscopy of Photosystem I. In Nanoimaging and Nanospectroscopy Iii; Verma, P., Egner, A., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2015; Volume 9554. [Google Scholar]
- Yang, M.; Damjanovic, A.; Vaswani, H.M.; Fleming, G.R. Energy transfer in photosystem I of cyanobacteria Synechococcus elongatus: Model study with structure-based semi-empirical Hamiltonian and experimental spectral density. Biophys.J. 2003, 85, 140–158. [Google Scholar] [CrossRef]
- van Stokkum, I.H.M.; Desquilbet, T.E.; van der Weij-de Wit, C.D.; Snellenburg, J.J.; van Grondelle, R.; Thomas, J.C.; Dekker, J.P.; Robert, B. Energy Transfer and Trapping in Red-Chlorophyll-Free Photosystem I from Synechococcus WH 7803. J. Phys. Chem. B 2013, 117, 11176–11183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sener, M.K.; Park, S.; Lu, D.Y.; Damjanovic, A.; Ritz, T.; Fromme, P.; Schulten, K. Excitation migration in trimeric cyanobacterial photosystem I. J. Chem. Phys. 2004, 120, 11183–11195. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Dahlbom, M.G.; Canfield, P.J.; Hush, N.S.; Kobayashi, R.; Reimers, J.R. Assignment of the Q(y) absorption spectrum of photosystem-i from Thermosynechococcus elongatus based on CAM-B3LYP calculations at the PW91-Optimized protein structure. J. Phys. Chem. B 2007, 111, 9923–9930. [Google Scholar] [CrossRef] [PubMed]
- Vaitekonis, S.; Trinkunas, G.; Valkunas, L. Red chlorophylls in the exciton model of photosystem I. Photosynth. Res. 2005, 86, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Pishchalnikov, R.Y.; Shubin, V.V.; Razjivin, A.P. Spectral differences between monomers and trimers of photosystem I depend on the interaction between peripheral chlorophylls of neighboring monomers in trimer. Phys. Wave Phenom. 2017, 25, 185–195. [Google Scholar] [CrossRef]
- Grotjohann, I.; Fromme, P. Structure of cyanobacterial Photosystem I. Photosynth. Res. 2005, 85, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, R.; Hayes, J.M.; Small, G.J. SPECTRAL Hole-burning spectroscopy in amorphous molecular-solids and proteins. Chem. Rev. 1993, 93, 1471–1502. [Google Scholar] [CrossRef]
- Pishchalnikov, R. Application of the differential evolution for simulation of the linear optical response of photosynthetic pigments. J. Comput. Phys. 2018, 372, 603–615. [Google Scholar] [CrossRef]
- Kubo, R.T.Y. Application of the Method of Generating Function to Radiative and Non-Radiative Transitions of a Trapped Electron in a Crystal. Prog. Theor. Phys. 1955, 13, 160–182. [Google Scholar] [CrossRef] [Green Version]
- Lax, M. The Franck-Condon Principle and Its Application to Crystals. J. Chem. Phys. 1952, 20, 1752–1760. [Google Scholar] [CrossRef]
- Zhang, W.M.; Meier, T.; Chernyak, V.; Mukamel, S. Exciton-migration and three-pulse femtosecond optical spectroscopies of photosynthetic antenna complexes. J. Chem. Phys. 1998, 108, 7763–7774. [Google Scholar] [CrossRef]
- Meier, T.; Chernyak, V.; Mukamel, S. Femtosecond photon echoes in molecular aggregates. J. Chem. Phys. 1997, 107, 8759–8780. [Google Scholar] [CrossRef]
- Yang, M.N.; Fleming, G.R. Influence of phonons on exciton transfer dynamics: Comparison of the Redfield, Forster, and modified Redfield equations. Chem. Phys. 2002, 275, 355–372. [Google Scholar] [CrossRef]
- Pishchalnikov, R.; Mueller, M.; Holzwarth, A. Theoretical modelling of the optical properties and the exciton dynamics of the isolated PSII reaction centre. Photosynth. Res. 2007, 91, 141. [Google Scholar]
Sample Availability: Not available. |









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pishchalnikov, R.; Shubin, V.; Razjivin, A. Single Molecule Fluorescence Spectroscopy of PSI Trimers from Arthrospira platensis: A Computational Approach. Molecules 2019, 24, 822. https://doi.org/10.3390/molecules24040822
Pishchalnikov R, Shubin V, Razjivin A. Single Molecule Fluorescence Spectroscopy of PSI Trimers from Arthrospira platensis: A Computational Approach. Molecules. 2019; 24(4):822. https://doi.org/10.3390/molecules24040822
Chicago/Turabian StylePishchalnikov, Roman, Vladimir Shubin, and Andrei Razjivin. 2019. "Single Molecule Fluorescence Spectroscopy of PSI Trimers from Arthrospira platensis: A Computational Approach" Molecules 24, no. 4: 822. https://doi.org/10.3390/molecules24040822
APA StylePishchalnikov, R., Shubin, V., & Razjivin, A. (2019). Single Molecule Fluorescence Spectroscopy of PSI Trimers from Arthrospira platensis: A Computational Approach. Molecules, 24(4), 822. https://doi.org/10.3390/molecules24040822