Photochemical Degradation of Cyanides and Thiocyanates from an Industrial Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Industrial Wastewater
2.3. Light Sources
- Italy
- db Electronic Instruments srl
- Via Teano, 2
- 20161 Milano
- Italy
- [Photonics]
- Sales:
- P: +39-02646-9341
- F: +39-02645-6632
- Support:
2.4. Photochemical and Photocatalytic Degradation Experiments
2.5. Analytical Methods
2.6. Toxicity Measurements
3. Results and Discussion
3.1. Characteristics of Light Sources
3.2. Photochemical Degradation
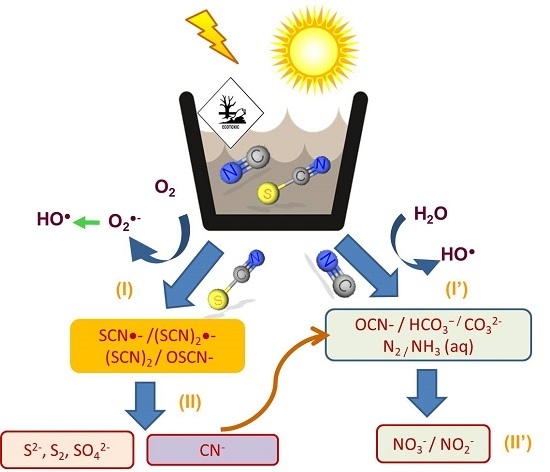
3.3. Reaction Mechanism
3.4. Evolution of the Toxicity of the Solutions during the Photocatalytic Runs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Botz, M.M.; Mudder, T.I.; Akcil, U.A. Cyanide treatment: Physical, chemical and biological processes. Develop. Miner. Process. 2005, 15, 672–702. [Google Scholar] [CrossRef]
- Donato, D.B.; Nichols, O.; Possingham, H.; Moore, M.; Ricci, P.F.; Nollera, B.N. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. J. Environ. Int. 2007, 33, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Soto, H.; Nava, F.; Leal, J.; Jarat, J. Regeneration of cyanide by ozone oxidation of thiocyanate in cyanidation tailings. Miner. Eng. 1995, 8, 273–281. [Google Scholar] [CrossRef]
- Gould, D.W.; King, M.; Mohapatra, B.R.; Cameron, R.A.; Kapoor, A.; Koren, D.W. A critical review on destruction of thiocyanate in mining effluents. Miner. Eng. 2012, 34, 38–47. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.O.; Utomi, C.E.; Mobo, M.; Mudumbi, J.B.; Ngongang, M.M.; Akinpelu, E.A. Performance of a continuously stirred tank bioreactor system connected in series for the biodegradation of thiocyanate and free cyanide. J. Environ. Chem. Eng. 2017, 5, 1936–1945. [Google Scholar] [CrossRef]
- Collado, S.; Laca, A.; Díaz, M. Catalytic wet oxidation of thiocyanate with homogeneous copper(II) sulphate Catalyst. J. Hazard. Mater. 2010, 177, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Collado, S.; Laca, A.; Díaz, M. Wet Oxidation of Thiocyanate under Different pH Conditions: Kinetics and Mechanistic Analysis. Ind. Eng. Chem. Res. 2009, 48, 9902–9909. [Google Scholar] [CrossRef]
- Christy, A.A.; Egeberg, P.K. Oxidation of thiocyanate by hydrogen peroxide-a reaction kinetic study by capillary electrophoresis. Talanta 2000, 51, 1049–1058. [Google Scholar] [CrossRef]
- Chung, J.; Wood, J.L. Oxidation of Thiocyanate to Cyanide and Sulfate by the Lactoperoxidase-Hydrogen Peroxide System. Arch. Biochem. Biophys. 1970, 141, 73–78. [Google Scholar] [CrossRef]
- Devuyst, E.A.; Conard, B.R.; Ettel, V.A. Pilot plant operation of the Inco SO2/air cyanide removal process. Can. Min. J. 1982, 8, 27–30. [Google Scholar]
- Sarla, M.; Pandit, M.; Tyagi, D.K.; Kapoor, J.C. Oxidation of cyanide in aqueous solution by chemical and photochemical process. J. Hazard. Mater. 2004, 116, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.; Amal, R.; Tran, T. Photocatalytic oxidation of cyanide: Kinetic and mechanistic studies. J. Mol. Catal. A Chem. 2003, 193, 285–297. [Google Scholar] [CrossRef]
- Bozzi, A.; Guasaquillo, I.; Kiwi, J. Accelerated removal of cyanides from industrial effluents by supported TiO2 photo-catalysts. Appl. Catal. B Environ. 2004, 51, 203–211. [Google Scholar] [CrossRef]
- Dabrowski, B.; Zaleska, A.; Janczarek, M.; Hupka, J.; Miller, J.D. Photo-oxidation of dissolved cyanide using TiO2 catalyst. J. Photochem. Photobiol. A Chem. 2002, 151, 201–205. [Google Scholar] [CrossRef]
- Bogidar, V.; Mihaylov, J.; Hendrix, L. Comparative catalytic activity of selected metal oxides and sulfides for the photo-oxidation of cyanide. J. Photochem. Photobiol. A Chem. 1993, 72, 173–177. [Google Scholar] [CrossRef]
- Draper, R.B.; Fox, M.A. Titanium DIoxide Photooxidation of Thiocyanate (SCN)2− Studied by Diffuse Reflectance Flash Photolysis. J. Phys. Chem. 1990, 94, 4628–4634. [Google Scholar] [CrossRef]
- Salmi, M.; Tkachenko, N.; Vehmanen, V.; Lamminmäki, R.J.; Karvinen, S.; Lemmetyinen, H. The effect of calcination on photocatalytic activity of TiO2 particles: Femtosecond study. J. Photochem. Photobiol. A Chem. 2004, 163, 395–401. [Google Scholar] [CrossRef]
- Kamat, P.V. Photoelectrochemistry in particulate systems. 3. Photo-transformations in the colloidal titania-thiocyanate system. Langmuir 1985, 1, 608–611. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 14403-1:2012. Water Quality—Determination of Total Cyanide and Free Cyanide using Flow Analysis (FIA and CFA); International Organization for Standardization: Geneva, Switzerland, 2012; pp. 1–14. [Google Scholar]
- Nagashima, S.; Ozawa, T. Spectrophotometric Determination of Cyanide with Isonicotinic Acid and Barbituric Acid. Int. J. Environ. Anal. Chem. 1981, 10, 99–106. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 11348-3:1998. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test); International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Kurashova, I.; Halevy, I.; Kamyshny, A. Kinetics of Decomposition of Thiocyanate in Natural Aquatic Systems. Environ. Sci. Technol. 2018, 52, 1234–1243. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.-T.; Yun, S.-T.; Kim, S.-O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 146, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Kim, T.; Jo, A.; Park, S.; Choi, K.; Zoh, K.-D. Degradation mechanism of cyanide in water using a UV-LED/H2O2/Cu2+ system. Chemosphere 2018, 208, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Pandit, M.; Kapoor, J.C.; Tyagi, D.K. Photo-oxidation of cyanide in aqueous solution by the UV/H2O2 proces. J. Chem. Technol. Biotechnol. 2005, 80, 13–19. [Google Scholar] [CrossRef]
- Wang, W.; Edwards, J.G.; Davies, J.A. Photooxidation of aqueous ammonia with titania-based heterogeneous catalysts. Solar Energy 1994, 52, 459–466. [Google Scholar] [CrossRef]
- Litter, M.; Blesa, M. Photodissolution of iron oxides 11: The lack of efficiency of thioycanate. Can. Chem. J. 1990, 68, 728–730. [Google Scholar] [CrossRef]
- Oulego, P.; Collado, S.; Laca, A.; Diaz, M. Simultaneous oxidation of cyanide and thiocyanate at high pressure and temperature. J. Hazard. Mater. 2014, 280, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Figlar, J.N.; Stanbury, D.M. Thiocyanogen as an intermediate in the oxidation of thiocyanate by hydrogen peroxide in acidic aqueous solution. Inorg. Chem. 2000, 39, 5089–5094. [Google Scholar] [CrossRef]
- Akcil, A. Destruction of cyanide in gold mill effluents: Biological versus chemical treatments. Biotechnol. Adv. 2003, 21, 501–511. [Google Scholar] [CrossRef]
- Weltens, R.; Deprez, K.; Michiels, L. Validation of Microtox as a first screening tool for waste classification. Waste Manag. 2014, 34, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Bilin, L.; Gmurek, M.; Ledakowicz, S. Comparison between industrial and simulated textile wastewater treatment by AOPs-Biodegradability, toxicity and cost assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar]
- Le, T.X.H.; Nguyen, T.V.; Yacouba, Z.A.; Zoungrana, L.; Avril, F.; Nguyen, D.L.; Petit, E.; Mendret, J.; Bonniol, V.; Bechelany, M.; et al. Correlation between degradation pathway and toxicity of acetaminophen and its by-products by using the electro-Fenton process in aqueous media. Chemosphere 2017, 172, 1–9. [Google Scholar] [PubMed]
- Marugan, J.; Bru, D.; Pablos, C.; Catala, M. Comparative evaluation of acute toxicity by Vibrio fischeri and fern spore based bioassays in the follow-up of toxic chemicals degradation by photocatalysis. J. Hazard. Mater. 2012. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.C.; Chu, A.; Goodman, H. Influence of salinity on Vibrio Fischeri and lux-modified pseudomonas fluorescens toxicity bioassay. Environ. Toxicol. Chem. 2000, 19, 2474–2477. [Google Scholar] [CrossRef]
Sample Availability: Sample of raw industrial wastewater is available from the author C.O.A. |





| Parameter | Value |
|---|---|
| pH | 8.6 |
| Conductivity (µS/cm, 20 °C) | 1067 |
| Suspended solids (mg/L) | 39 |
| Chemical oxygen demand (mg/L) | 848 |
| Total organic carbon (mg/L) | 291 |
| Phenols (mg/L) | 4.2 |
| Total hydrocarbons (mg/L) | 2.2 |
| Thiocyanates (mg/L) | 226 |
| Total cyanides (mg/L) | 105 |
| Wavelength (nm) | Irradiance (mW/cm2) | Remarks | |
|---|---|---|---|
| UVC | 254 | 26 | Low pressure Hg lamp |
| UV-Vis | 200–600 | 52 | High pressure Hg lamp |
| SimSolar | 200–800 | 190 | Xe lamp, simulated solar light |
| Lamp | Illumination Time | SCN− to SO42− | SCN− and CN− to N-species |
|---|---|---|---|
| UVC | 1 h | 0.99 | 0.81 |
| UVC | 3 h | 0.99 | 0.97 |
| UVC | 6 h | 0.99 | 0.99 |
| UV-Vis | 1 h | 0.95 | 0.82 |
| UV-Vis | 3 h | 0.99 | 0.87 |
| UV-Vis | 6 h | 0.99 | 0.96 |
| SimSolar | 1 h | 0.94 | 0.99 |
| SimSolar | 3 h | 0.95 | 0.99 |
| SimSolar | 6 h | 0.99 | 0.89 |
| SimSolar + TiO2 | 1 h | 0.98 | 0.92 |
| SimSolar + TiO2 | 3 h | 0.99 | 0.86 |
| SimSolar + TiO2 | 6 h | 0.99 | 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viña Mediavilla, J.J.; Fernandez Perez, B.; Fernandez de Cordoba, M.C.; Ayala Espina, J.; Ania, C.O. Photochemical Degradation of Cyanides and Thiocyanates from an Industrial Wastewater. Molecules 2019, 24, 1373. https://doi.org/10.3390/molecules24071373
Viña Mediavilla JJ, Fernandez Perez B, Fernandez de Cordoba MC, Ayala Espina J, Ania CO. Photochemical Degradation of Cyanides and Thiocyanates from an Industrial Wastewater. Molecules. 2019; 24(7):1373. https://doi.org/10.3390/molecules24071373
Chicago/Turabian StyleViña Mediavilla, Juan Jose, Begoña Fernandez Perez, Maria C. Fernandez de Cordoba, Julia Ayala Espina, and Conchi O. Ania. 2019. "Photochemical Degradation of Cyanides and Thiocyanates from an Industrial Wastewater" Molecules 24, no. 7: 1373. https://doi.org/10.3390/molecules24071373
APA StyleViña Mediavilla, J. J., Fernandez Perez, B., Fernandez de Cordoba, M. C., Ayala Espina, J., & Ania, C. O. (2019). Photochemical Degradation of Cyanides and Thiocyanates from an Industrial Wastewater. Molecules, 24(7), 1373. https://doi.org/10.3390/molecules24071373