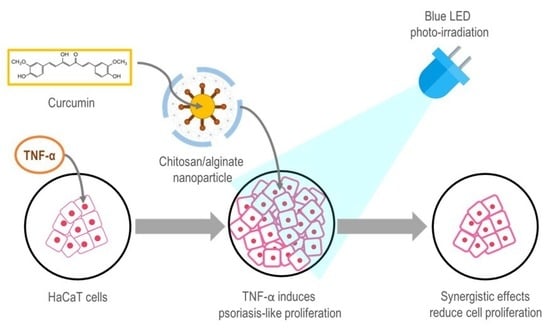
Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Cur-CS/Alg NP Formulation
2.2. Optimization of the Blue LED-Based Illumination Device
2.3. In Vitro Cytotoxicity/Anti-Proliferation Effect of the PDT on Normal and Tumor Necrosis Factor-Alpha (TNF-α)-Induced Cultured Human Kerlatinocyte (HaCaT) Cells
2.4. Physical Stability
2.5. In Vitro Cellular Uptake
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Cur-CS/Alg NPs
3.3. Characterization of the Cur-CS/Alg NPs
3.4. Design and Optimization of the Cur-CS/Alg NP Formulation
3.5. Fabrication of the Blue LED Based Illumination Device
3.6. Cell Culture
3.7. Induction of Proliferation of HaCaT Cells by TNF-α
3.8. Cell Treatment and Photo-Irradiation
3.9. Cell Viability Assay
3.10. Physical Stability in Cell Culture Medium
3.11. In Vitro Cellular Uptake
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Report on Psoriasis 2016; World Health Organization: Geneva, Switzerland, 2016.
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet. 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Higgins, E. Psoriasis. Medicine 2017, 45, 368–378. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy. Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, D.H.; Park, H.J. Molecular mechanisms and management of a cutaneous inflammatory disorder: Psoriasis. Int J. Mol. Sci. 2017, 18, 2684. [Google Scholar] [CrossRef]
- Lapolla, W.; Yentzer, B.A.; Bagel, J.; Halvorson, C.R.; Feldman, S.R. A review of phototherapy protocols for psoriasis treatment. J. Am. Acad. Dermatol. 2011, 64, 936–949. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Cai, Q.; Ren, Q.; Wei, L. Red light combined with blue light irradiation regulates proliferation and apoptosis in skin keratinocytes in combination with low concentrations of curcumin. PLoS ONE 2015, 10, E0138754. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Invest. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf. B Biointerfaces. 2015, 128, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, H.W.; Yuan, K.H.; Li, F.L.; Huang, Z. Combination of photodynamic therapy and immunomodulation for skin diseases--update of clinical aspects. Photochem. Photobiol. Sci. 2011, 10, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Larisch, P.; Verwanger, T.; Linecker, M.; Krammer, B. The interrelation between a pro-inflammatory milieu and fluorescence diagnosis or photodynamic therapy of human skin cell lines. Photodiagnosis Photodyn. Ther. 2014, 11, 91–103. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Ratnatilaka Na Bhuket, P.; El-Magboub, A.; Haworth, I.S.; Rojsitthisak, P. Enhancement of curcumin bioavailability via the prodrug approach: Challenges and prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Wongsrisakul, J.; Wichitnithad, W.; Rojsitthisak, P.; Towiwat, P. Antinociceptive effects of curcumin diethyl disuccinate in animal models. J. Health Res. 2010, 24, 175–180. [Google Scholar]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Muangnoi, C.; Jithavech, P.; Ratnatilaka Na Bhuket, P.; Supasena, W.; Wichitnithad, W.; Towiwat, P.; Niwattisaiwong, N.; Haworth, I.S.; Rojsitthisak, P. A curcumin-diglutaric acid conjugated prodrug with improved water solubility and antinociceptive properties compared to curcumin. Biosci. Biotechnol. Biochem. 2018, 82, 1301–1308. [Google Scholar] [CrossRef]
- Bhunchu, S.; Rojsitthisak, P. Biopolymeric alginate-chitosan nanoparticles as drug delivery carriers for cancer therapy. Pharmazie 2014, 69, 563–570. [Google Scholar] [PubMed]
- Bhunchu, S.; Rojsitthisak, P.; Rojsitthisak, P. Effects of preparation parameters on the characteristics of chitosan–alginate nanoparticles containing curcumin diethyl disuccinate J. Drug Deliv. Sci. Technol. 2015, 28, 64–72. [Google Scholar] [CrossRef]
- Bhunchu, S.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Curcumin diethyl disuccinate encapsulated in chitosan/alginate nanoparticles for improvement of its in vitro cytotoxicity against MDA-MB-231 human breast cancer cells. Pharmazie 2016, 71, 691–700. [Google Scholar]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C. Mater. Biol. Appl. 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Fattahpour, S.; Shamanian, M.; Tavakoli, N.; Fathi, M.; Sheykhi, S.R.; Fattahpour, S. Design and optimization of alginate-chitosan-pluronic nanoparticles as a novel meloxicam drug delivery system. J. Appl. Polym. Sci. 2015, 132, 42241. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Tracking the transdermal penetration pathways of optimized curcumin-loaded chitosan nanoparticles via confocal laser scanning microscopy. Int. J. Biol. Macromol. 2018, 108, 753–764. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Noomun, K.; Jongaroonngamsang, N.; Rojsitthisak, P.; Nimmannit, U. Preparation of alginate nanocapsules containing turmeric oil. Carbohydr. Polym. 2008, 74, 209–214. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mater. Sci. Eng. C. 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, H.; Huang, Z.; Lin, H.; Ke, Z.; Xie, S.; Li, B. Light-emitting diode-based illumination system for in vitro photodynamic therapy. Int. J. Photoenergy 2012, 2012, 920671. [Google Scholar] [CrossRef]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and anti-inflammatory activity of chitosan-alginate nanoparticles: A targeted therapy for cutaneous pathogens. J. Invest. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef]
- Chopra, D.; Ray, L.; Dwivedi, A.; Tiwari, S.K.; Singh, J.; Singh, K.P.; Kushwaha, H.N.; Jahan, S.; Pandey, A.; Gupta, S.K.; et al. Photoprotective efficiency of PLGA-curcumin nanoparticles versus curcumin through the involvement of ERK/AKT pathway under ambient UV-R exposure in HaCaT cell line. Biomaterials. 2016, 84, 25–41. [Google Scholar] [CrossRef]
Sample Availability: Curcumin is available from the authors. |






| Run | Factor | Response | |||||
|---|---|---|---|---|---|---|---|
| X1 (mg/mL) | X2 (% w/v) | X3 | Y1 (nm) | Y2 (mV) | Y3 (%) | Y4 (%) | |
| 1 | 0.5 | 0.5 | 0.1:1 | 282 ± 63 | −22.3 ± 5.7 | 46.9 ± 0.2 | 7.1 ± 1.6 |
| 2 | 1.5 | 0.5 | 0.1:1 | 263 ± 57 | −24.3 ± 1.8 | 49.0 ± 0.6 | 24.0 ± 1.3 |
| 3 | 0.5 | 2 | 0.1:1 | 330 ± 14 | −22.1 ± 4.4 | 45.7 ± 0.2 | 4.7 ± 1.8 |
| 4 | 1.5 | 2 | 0.1:1 | 294 ± 46 | −20.6 ± 2.2 | 48.8 ± 0.3 | 14.6 ± 1.0 |
| 5 | 0.5 | 1 | 0.05:1 | 295 ± 61 | −11.4 ± 2.5 | 48.4 ± 0.2 | 9.9 ± 5.9 |
| 6 | 1.5 | 1 | 0.05:1 | 281 ± 34 | −10.8 ± 5.2 | 54.5 ± 1.6 | 27.4 ± 9.6 |
| 7 | 0.5 | 1 | 0.2:1 | 984 ± 80 | −27.4 ± 1.4 | 48.9 ± 0.1 | 4.0 ± 0.7 |
| 8 | 1.5 | 1 | 0.2:1 | 1120 ±8 4 | −28.4 ± 1.6 | 48.2 ± 0.4 | 12.5 ± 2.9 |
| 9 | 1 | 0.5 | 0.05:1 | 267 ± 52 | −14.3 ± 1.1 | 49.2 ± 0.8 | 10.9 ± 3.7 |
| 10 | 1 | 2 | 0.05:1 | 199 ± 37 | −13.5 ± 6.0 | 49.5 ± 1.5 | 18.1 ± 4.1 |
| 11 | 1 | 0.5 | 0.2:1 | 899 ± 6 | −30.8 ± 1.4 | 46.9 ± 0.6 | 7.6 ± 3.4 |
| 12 | 1 | 2 | 0.2:1 | 992 ± 180 | −25.4 ± 1.6 | 46.0 ± 0.2 | 6.0 ± 2.2 |
| 13 | 1 | 1 | 0.1:1 | 255 ± 88 | −25.0 ± 2.2 | 48.8 ± 0.3 | 11.9 ± 5.1 |
| 14 | 1 | 1 | 0.1:1 | 245 ± 102 | −22.4 ± 1.0 | 49.2 ± 0.9 | 12.3 ± 5.4 |
| 15 | 1 | 1 | 0.1:1 | 287 ± 37 | −21.8 ± 0.8 | 47.8 ± 1.1 | 11.8 ± 4.3 |
| Response | Model | Sequential p-Value | Lack of Fit p-Value | Adjusted R2 | Predicted R2 | Remark |
|---|---|---|---|---|---|---|
| Y1 (Particle size) | Linear | <0.0001 | 0.0198 | 0.8251 | 0.7453 | |
| 2FI | 0.9273 | 0.0140 | 0.7723 | 0.4327 | ||
| Quadratic | 0.0004 | 0.2076 | 0.9882 | 0.9236 | Suggested | |
| Y2 (Zeta potential) | Linear | 0.0005 | 0.2065 | 0.7263 | 0.5988 | |
| 2FI | 0.8404 | 0.1569 | 0.6591 | 0.1750 | ||
| Quadratic | 0.0030 | 0.8698 | 0.9594 | 0.9002 | Suggested | |
| Y3 (EE) | Linear | 0.0520 | 0.1363 | 0.3517 | −0.0626 | |
| 2FI | 0.2574 | 0.1471 | 0.4471 | −0.7347 | ||
| Quadratic | 0.0082 | 0.6047 | 0.9010 | 0.6598 | Suggested | |
| Y4 (LC) | Linear | 0.0002 | 0.0063 | 0.7726 | 0.6473 | Suggested |
| 2FI | 0.3127 | 0.0064 | 0.7948 | 0.4137 | ||
| Quadratic | 0.6684 | 0.0042 | 0.7534 | −0.9670 |
| Factors | Y1 (Particle size) | Y2 Zeta potential | Y3 (EE) | Y4 (LC) |
|---|---|---|---|---|
| X1 (Curcumin concentration) | 0.3544 | 0.9619 | 0.0062 | 0.0001 |
| X2 (Tween® 80 concentration) | 0.2315 | 0.0218 | 0.2860 | 0.4089 |
| X3 (CS/Alg mass ratio) | <0.0001 | <0.0001 | 0.0015 | 0.0023 |
| Optimized Formulation (X1, X2, X3) | Response | Predicted Value | Observed Value | Error * |
|---|---|---|---|---|
| 15 mg/mL, 0.5% (w/v), 0.08:1 | Y1 (nm) | 254 | 245 ± 11 | 3.6 |
| Y2 (mV) | −20.2 | −21.1 ± 1.2 | 4.2 | |
| Y3 (%) | 50.4 | 47.6 ± 1.8 | 5.5 | |
| Y4 (%) | 21.5 | 22.8 ± 0.5 | 6.1 | |
| * Error was calculated as | ||||
| Level | ||||
|---|---|---|---|---|
| Low | Medium | High | ||
| Independent variables (factors) | ||||
| X1 = Curcumin concentration (mg/mL) | 0.5 | 1 | 1.5 | |
| X2 = Tween® 80 concentration (% w/v) | 0.5 | 1 | 2 | |
| X3 = CS/Alg mass ratio | 0.05:1 | 0.1:1 | 0.2:1 | |
| Dependent variables (responses) | Constraints | |||
| Y1 = Particle size (nm) | 200–300 nm | |||
| Y2 = Zeta potential (mV) | −30 mV to −20 mV | |||
| Y3 = EE (%) | Maximize | |||
| Y4 = LC (%) | Maximize | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes. Molecules 2019, 24, 1388. https://doi.org/10.3390/molecules24071388
Gomez C, Muangnoi C, Sorasitthiyanukarn FN, Wongpiyabovorn J, Rojsitthisak P, Rojsitthisak P. Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes. Molecules. 2019; 24(7):1388. https://doi.org/10.3390/molecules24071388
Chicago/Turabian StyleGomez, Clinton, Chawanphat Muangnoi, Feaungthit Niyamissara Sorasitthiyanukarn, Jongkonnee Wongpiyabovorn, Pornchai Rojsitthisak, and Pranee Rojsitthisak. 2019. "Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes" Molecules 24, no. 7: 1388. https://doi.org/10.3390/molecules24071388
APA StyleGomez, C., Muangnoi, C., Sorasitthiyanukarn, F. N., Wongpiyabovorn, J., Rojsitthisak, P., & Rojsitthisak, P. (2019). Synergistic Effects of Photo-Irradiation and Curcumin-Chitosan/Alginate Nanoparticles on Tumor Necrosis Factor-Alpha-Induced Psoriasis-Like Proliferation of Keratinocytes. Molecules, 24(7), 1388. https://doi.org/10.3390/molecules24071388