A Low Glycemic Index Decreases Inflammation by Increasing the Concentration of Uric Acid and the Activity of Glutathione Peroxidase (GPx3) in Patients with Polycystic Ovary Syndrome (PCOS)
Abstract
:1. Introduction
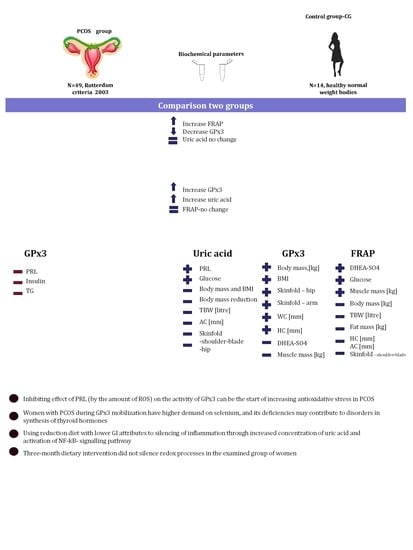
2. Results
3. Discussion
4. Material and Methods
4.1. Participants
- -
- Women with PCOS vs. the control group,
- -
- Women with PCOS vs. women with PCOS after dietary intervention,
- -
- Women with PCOS after dietary intervention vs control group.
4.2. Anthropometric Measurements
4.3. Blood Sample Collection
4.4. Biochemical Measurements
4.5. The Ferric Reducing Ability of Plasma (FRAP)
4.6. Uric Acid Assay in Blood Plasma
4.7. Glutathione Peroxidase Assay in Blood Plasma
4.8. Dietary Intervention
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GPx3 | glutathione peroxidase |
| FRAP | ferric reducing/antioxidant power |
| PCOS | polycystic ovary syndrome |
| TG | triglycerides |
| DHEA-SO4 | dehydroepiandrosterone sulphate |
| PRL | prolactin |
| AC | arm circumference |
| WC | waist Circumference |
| HC | hip circumference |
| FM | fat mass |
| TBW | total body water |
| BMI | body mass index |
| WHR | waist-hip ratio |
References
- Szczuko, M.; Malarczyk, I.; Zapalowska-Chwyć, M. Improvement in anthropometric parameters after rationally dietary intervention in women with polycystic ovary syndrome as the best method to support treatment. Roczniki Państwowego Zakładu Higieny 2017, 68, 409–417. [Google Scholar] [PubMed]
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Fenkci, V.; Fenkci, S.; Yilmazer, M.; Serteser, M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Sterility 2003, 80, 123–127. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, D. The role of oxidative stress in the pathogenesis of polycystic ovary syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban 2012, 43, 187–190. [Google Scholar] [PubMed]
- Desai, V.; Prasad, N.R.; Manohar, S.M.; Sachan, A.; Venkata, S.R.P.; Narasimha, L. Oxidative Stress in Non-Obese Women with Polycystic Ovarian Syndrome. J. Clin. Diagn. Res. 2014, 8, CC01–CC03. [Google Scholar]
- Szczuko, M.; Skowronek, M.; Zapałowska-Chwyć, M.; Starczewski, A. Quantitive assessment of nutrition In patients with polycystic ovary syndrome (PCOS). Roczniki Państwowego Zakładu Higieny 2016, 67, 419–426. [Google Scholar] [PubMed]
- Szczuko, M.; Sankowska, M.; Zapałowska-Chwyć, M.; Wysokiński, P. Studies on the quality nutrition in women with polycystic ovary syndrome (PCOS). Roczniki Państwowego Zakładu Higieny 2017, 68, 61–67. [Google Scholar] [PubMed]
- Zhao, Y.; Zhang, C.; Huang, Y.; Yu, Y.; Li, R.; Li, M.; Liu, N.; Liu, P.; Qiao, J. Up-Regulated Expression of WNT5a Increases Inflammation and Oxidative Stressvia PI3K/AKT/NF-κB Signaling in the Granulosa Cells of PCOS Patients. J. Clin. Endocrinol. Metab. 2015, 100, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallan, P.; Carrascosa, A.; Gussinye, M.; Dominguez, C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Rad. Biol. Med. 2003, 34, 1563–1574. [Google Scholar] [CrossRef]
- Tonstad, S. Cigarette smoking, smoking cessation, and diabetes. Diabetes Res. Clin. Pract. 2009, 85, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M. The role of disorders of the prooxidant-antioxidant system in diabetes etiopathology. Postepy higieny i medycyny doswiadczalnej 2011, 65, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Flekac, M.; Skrha, J.; Hilgertova, J.; Lacinova, Z.; Jarolimkova, M. Gene polymorphisms of superoxide dismutases and catalase in diabetes mellitus. BMC Med. Genet. 2008, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, J.; Jennings, L.K. Leukocyte-derived myeloperoxidase amplifies high-glucose-induced endothelial dysfunction through interaction with high-glucose-stimulated, vascular non-leukocyte-derived reactive oxygen species. Diabetes 2004, 53, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; de Marañon, A.M.; Rios-Navarro, C.; Alvarez, A.; Gomez, M.; Rocha, M.; Hernández-Mijares, A. Insulin Resistance in PCOS Patients Enhances Oxidative Stress and Leukocyte Adhesion: Role of Myeloperoxidase. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hatley, M.E.; Bolick, D.T.; Palmer, L.A.; Edelstein, D.; Brownlee, M.; Hedrick, C.C. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelia cells. Diabetologia 2004, 47, 1727–1734. [Google Scholar] [CrossRef]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Gutowicz, M. Effects of reactive oxygen species on central nervous system. Postępy Hig. Med. Dośw 2011, 65, 104–113. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Szczuko, M.; Zapałowska-Chwyć, M.; Drozd, A.; Maciejewska, D.; Starczewski, A.; Stachowska, E. Effect of IGF-I and TNF-alpha on intensification of steroid pathways in women with PCOS phenotypes are not identical. Enhancement of progesterone pathway in women with PCOS increases the concentration of TNF-alpha. Gynecol. Endocrinol. 2016, 32, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, A.; Gromadzka, G. Oxidative stress and natural antioxidant mechanisms: The role in neurodegeneration. From molecular mechanisms to therapeutic strategies. Postepy Hig. Med. Dosw. 2013, 67, 43–53. [Google Scholar]
- Łukasiewicz-Hussain, A. The role of gluthatione and gluthatione-related enzymes in antioxidative processes. Medycyna. Pracy. 2003, 54, 473–479. [Google Scholar]
- Sautin, Y.Y.; Johnoson, J.R. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Georgieva, L.; Mihaylova, D. Screening of total phenolic content and radical scavenging capacity of Bulgarian plant species. Int. Food Res. J. 2015, 22, 240–245. [Google Scholar]
- Mihaylova, D.; Schalow, S. Antioxidant and Stabilization Activity of a Quercetin-Containing Flavonoid Extract Obtained from Bulgarian Sophora japonica L. Braz. Arch. Biol. Technol. 2013, 56, 431–438. [Google Scholar] [CrossRef]
- Szczuko, M.; Zapałowska-Chwyć, M.; Maciejewska, D.; Drozd, A.; Starczewski, A.; Stachowska, E. Significant improvement selected mediators of inflammation in phenotypes of women with PCOS after reduction and low GI diet. Mediators Inflammation 2017, 5489523. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Cai, X.; Li, X.; Li, L.; Huang, H.Z.; Liu, Y.S.; Chen, L.; Zhang, K.E.; Wang, L.; Li, X.; Song, J.; et al. Adiponectin reduces carotid atherosclerotic plaque formation in ApoE-/-mice: Roles of oxidative and nitrosative stress and inducible nitric oxide synthase. Mol. Med. Rep. 2015, 11, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Lam, K.S.; Wang, B.; Xu, A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pu, H.; Ma, C.; Jiang, T.; Wei, Q.; Zhang, C.; Duan, M.; Shou, X.; Su, L.; Zhang, J.; et al. Adiponectin abates atherosclerosis by reducing oxidative stress. Med. Sci. Monit. 2014, 20, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Comim, F.V.; Gutierrez, K.; Bridi, A.; Bochi, G.; Chemeris, R.; Rigo, M.L.; Dau, A.M.P.; Cezar, A.S.; Moresco, R.N.; Gonçalves, P.B.D. Effects of adiponectin including reduction of androstenedione secretion and ovarian oxidative stress parameters in vivo. PLoS ONE 2016, 11, e0154453. [Google Scholar] [CrossRef] [PubMed]
- Klocek-Gorka, B.; Szczesna, M.; Molik, E.; Zieba, D.A. The interactions of season, leptin and melatonin levels with thyroid hormone secretion, using an in vitro approach. Small Ruminant Res. 2010, 91, 231–235. [Google Scholar] [CrossRef]
- Veena, B.S.; Sharmila, U.; Satish, K.A.; Pratap, K.N. Evaluation of oxidative stress, antioxidants and prolactin in infertile women. Indian J. Clin. Biochem. 2008, 23, 186–190. [Google Scholar] [CrossRef]
- Baszczuk, A.; Kopczyński, Z.; Thielemann, A. Endothelial dysfunction in patients with primary hypertension and hyperhomocysteinemia. Postepy Hig. Med. Dosw. 2014, 68, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.H.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress. Potential role in fructose –dependent and independent fatty liver. J. Biol Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Jia, L.; Xing, J.; Ding, Y.; Shen, Y.; Shi, X.; Ren, W.; Wan, M.; Guo, J.; Zheng, S.; Liu, Y.; et al. Hyperuricemia Causes Pancreatic β-Cell Death and Dysfunction through NF-κB Signaling Pathway. PLoS ONE 2013, 8, e78284. [Google Scholar] [CrossRef]
- Jacob, M.H.; Janner, D.R.; Araújo, A.S.; Jahn, M.P.; Kucharski, L.C.; Moraes, T.B.; Dutra Filho, D.C.; Ribeiro, M.F.; Belló-Klein, A. Dehydroepiandrosterone improves hepatic antioxidant reserve and stimulates akt signaling in young and old rats. J. Steroid Biochem. Mol. Biol. 2011, 13, 331–336. [Google Scholar] [CrossRef]
- Blair, S.A.; Kyaw-Tun, T.; Young, I.S.; Phelan, N.A.; Gibney, J.; McEneny, J. Oxidative stress and inflammation in lean and obese subjects with polycystic ovary syndrome. J. Reprod. Med. 2013, 58, 107–114. [Google Scholar]
- Szczuko, M.; Zapałowska-Chwyć, M.; Maciejewska, D.; Drozd, A.; Starczewski, A.; Stachowska, E. High glycemic index diet in PCOS patients. The analysis of IGF I and TNF-alpha pathways in metabolic disorders. Med. Hypotheses 2016, 96, 42–47. [Google Scholar] [CrossRef]
- Singla, R.; Gupta, Y.; Khemani, M.; Aggarwal, S. Thyroid disorders and polycystic ovary syndrome: An emerging relationship. Indian J. Endocrinol. Metab. 2015, 19, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E. Diagnosis of polycystic ovary syndrome: From NIH criteria to ESHRE-ASRM guidelines. Minerva. Ginecol. 2004, 56, 1–6. [Google Scholar] [PubMed]
- Railly, J.J.; Wilson, J.; Durnin, J.U.G.A. Determination of body composition from skin fold thickness: A validation study. Arch. Dis. Child. 1995, 73, 305–310. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tietz, N.W. Text Book of Clinical Chemistry, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 1999; pp. 809–861. [Google Scholar]
- Flohé, L.; Gunzler, W.A. Assay of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [PubMed]
- Kunachowicz, H.; Nadolna, I.; Przygoda, B.; Iwanow, K. Tabele składu i wartości odżywczych żywności; PZWL: Warszawa, Poland, 2005. [Google Scholar]
Sample Availability: Not available. |



| Parameter | Average Values in Groups | Significance of Differences | ||||
|---|---|---|---|---|---|---|
| PCO I | PCO II | CG | I vs. II | I vs. CG | II vs. CG | |
| Uric acid mg/dL | 4.58 ± 1.65 | 7.40 ± 1.61 | 4.91 ± 2.72 | 0.000 | 0.542 | 0.001 |
| FRAP umol/L | 1093.1 ± 327.1 | 1041.0 ± 221.5 | 818.7 ± 263.4 | 0.541 | 0.001 | 0.005 |
| GPx3 mU/mL | 555.3 ± 220.4 | 858.2 ± 326.7 | 871.4 ± 360.3 | 0.000 | 0.000 | 0.902 |
| Correlation Matrix | Uric Acid | FRAP | GPx3 |
|---|---|---|---|
| Height | 0.039 | 0.004 | −0.326 |
| BM–no clothes | −0.245 | −0.138 | −0.202 |
| BMR | −0.161 | −0.211 | −0.003 |
| BMI | −0.273 | −0.153 | −0.098 |
| DHEA-SO4 | −0.197 | 0.009 | −0.213 |
| Androstenedione | 0.240 | −0.415 | 0.458 |
| TSH | 0.238 | −0.014 | −0.155 |
| LH | 0.290 | 0.036 | 0.033 |
| FSH | −0.072 | 0.203 | 0.374 |
| Oestradiol | 0.090 | −0.188 | 0.225 |
| SHBG | 0.349 | 0.127 | 0.116 |
| Testosterone | 0.432 | −0.101 | 0.097 |
| Prolactin | −0.417 | 0.021 | −0.656 |
| Insulin 0 | −0.002 | −0.017 | −0.572 |
| Insulin after 2 h | −0.200 | −0.048 | −0.210 |
| Glucose | −0.460 | −0.116 | −0.059 |
| Glucose after 2 h | −0.520 | −0.360 | 0.163 |
| Cholesterol | −0.265 | 0.008 | −0.419 |
| LDL | −0.061 | −0.039 | −0.163 |
| TG | −0.444 | −0.011 | −0.557 |
| HDL | 0.271 | 0.092 | 0.314 |
| Parameters | Uric Acid | FRAP | GPx3 |
|---|---|---|---|
| Body mass | −0.833 | −0.968 | 0.787 |
| Body mass reduction | −0.922 | −0.587 | 0.308 |
| BMR | −0.540 | −0.151 | 0.345 |
| CPM | −0.540 | −0.151 | 0.345 |
| Na/K | 0.142 | −0.424 | −0.243 |
| TBW % | 0.154 | 0.172 | −0.496 |
| TBW IN % | −0.024 | 0.543 | 0.040 |
| TBW EX % | 0.024 | −0.543 | −0.040 |
| TBW litre | −0.662 | −0.776 | 0.444 |
| TBW IN litre | −0.561 | −0.189 | 0.368 |
| TBW EX litre | −0.363 | −0.845 | 0.251 |
| Phase angle PA | −0.075 | 0.482 | 0.072 |
| fat mass % | −0.159 | −0.183 | 0.503 |
| fat mass kg | −0.606 | −0.694 | 0.792 |
| BCM kg | −0.542 | −0.151 | 0.346 |
| BCM % | −0.036 | 0.531 | 0.052 |
| Muscle mass kg | 0.285 | 0.723 | −0.756 |
| Muscle mass % | 0.566 | 0.888 | −0.877 |
| Waist circumference | −0.447 | −0.411 | 0.932 |
| Hip circumference | −0.583 | −0.827 | 0.699 |
| Arm circumference | −0.783 | −0.809 | 0.626 |
| Skinfold–shoulder-blade | −0.828 | −0.712 | 0.388 |
| Skinfold–hip | −0.904 | −0.506 | 0.712 |
| Skinfold–arm | −0.314 | −0.500 | 0.912 |
| BMI | −0.786 | −0.385 | 0.765 |
| WHR | 0.251 | 0.613 | 0.075 |
| DHEA-SO4 | 0.296 | 0.701 | −0.632 |
| Androstenedione | 0.297 | 0.228 | 0.407 |
| LH | 0.194 | −0.164 | 0.580 |
| FSH | −0.443 | 0.062 | 0.540 |
| Oestradiol | 0.520 | −0.102 | −0.382 |
| SHBG | 0.128 | −0.257 | −0.486 |
| Testosterone | 0.334 | −0.372 | −0.056 |
| Prolactin | 0.782 | 0.203 | −0.197 |
| Insulin sample 0 | 0.337 | −0.138 | 0.545 |
| Insulin after 2 h | −0.600 | −0.334 | 0.057 |
| Glucose | 0.966 | 0.783 | −0.468 |
| Glucose after 2 h | −0.146 | 0.466 | −0.392 |
| Cholesterol | −0.223 | 0.163 | 0.455 |
| LDL | −0.337 | 0.242 | 0.292 |
| TG | 0.247 | 0.158 | 0.544 |
| HDL | 0.285 | 0.0156 | −0.396 |
| Parameter | Avg | SD |
|---|---|---|
| Height m | 1.67 | 0.06 |
| Body mass kg | 82.97 | 17.32 |
| BMR kcal | 1508.66 | 146.15 |
| BMI kg/m2 | 29.68 | 6.48 |
| DHEA-SO4 µg/dL | 243.29 | 91.44 |
| Androstenedione ng/mL | 3.64 | 1.67 |
| TSH mIU/mL | 1.70 | 0.77 |
| LH mIU/mL | 7.71 | 3.62 |
| FSH mIU/mL | 5.03 | 0.95 |
| Oestradiol pg/mL | 45.19 | 24.68 |
| SHBG nmol/L | 35.05 | 16.39 |
| Testosterone ng/mL | 0.73 | 0.96 |
| Prolactin ng/mL | 17.63 | 7.25 |
| Insulin 0 mU/L | 13.98 | 10.88 |
| Insulin after 2 h mU/L | 79.80 | 47.97 |
| Glucose mg/dL | 91.82 | 10.87 |
| Glucose after 2 h mg/dL | 114.53 | 24.45 |
| Cholesterol mg/dL | 179.21 | 30.72 |
| LDL mg/dL | 111.82 | 31.42 |
| TG mg/dL | 102.53 | 52.41 |
| HDL mg/dL | 55.89 | 18.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczuko, M.; Zapalowska-Chwyć, M.; Drozd, R. A Low Glycemic Index Decreases Inflammation by Increasing the Concentration of Uric Acid and the Activity of Glutathione Peroxidase (GPx3) in Patients with Polycystic Ovary Syndrome (PCOS). Molecules 2019, 24, 1508. https://doi.org/10.3390/molecules24081508
Szczuko M, Zapalowska-Chwyć M, Drozd R. A Low Glycemic Index Decreases Inflammation by Increasing the Concentration of Uric Acid and the Activity of Glutathione Peroxidase (GPx3) in Patients with Polycystic Ovary Syndrome (PCOS). Molecules. 2019; 24(8):1508. https://doi.org/10.3390/molecules24081508
Chicago/Turabian StyleSzczuko, Małgorzata, Marta Zapalowska-Chwyć, and Radosław Drozd. 2019. "A Low Glycemic Index Decreases Inflammation by Increasing the Concentration of Uric Acid and the Activity of Glutathione Peroxidase (GPx3) in Patients with Polycystic Ovary Syndrome (PCOS)" Molecules 24, no. 8: 1508. https://doi.org/10.3390/molecules24081508
APA StyleSzczuko, M., Zapalowska-Chwyć, M., & Drozd, R. (2019). A Low Glycemic Index Decreases Inflammation by Increasing the Concentration of Uric Acid and the Activity of Glutathione Peroxidase (GPx3) in Patients with Polycystic Ovary Syndrome (PCOS). Molecules, 24(8), 1508. https://doi.org/10.3390/molecules24081508