Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest
Abstract
:1. Introduction
2. Results
2.1. Essential Oils Yield and Density
2.2. Chemical Composition
2.2.1. DPPH and ABTS Assays
2.2.2. PCL Photochemiluminescence
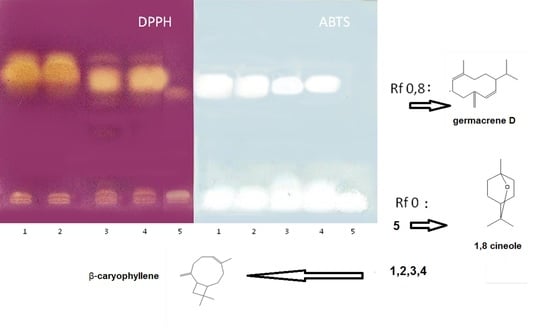
2.2.3. HPTLC Antiradical Bioautographic Assay with DPPH and ABTS
2.3. Evaluation of the Minimum Inhibitory Concentration (MIC)
Bioautographic Antibacterial Activity of the Essential Oil of H. coronarium
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. GC-MS and GC-FID Analyses
4.3. Antioxidant Activity
4.3.1. Quantitative Free Radical Scavenging Activity: DPPH and ABTS Assays
4.3.2. Photochemiluminscence Assay
4.3.3. Qualitative Radical Scavenging Activity: HPTLC Bioautographic Assay
4.4. Antimicrobial Activity: Evaluation of the Minimum Inhibitory Concentration
Bioautographic Antimicrobial Activity of H. coronarium Essential Oil
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arguello, M.A. Introducción a la Flora y Fauna del Ecuador, 1st ed.; PROPAD Universidad Tecnológica Equinoccial: Quito, Ecuador, 2008; pp. 5–7. [Google Scholar]
- De la Torre, L.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador, 1st ed.; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador and Herbario AAU Department of Biological Sciences, University of Aathus: Quito, Ecuador, 2008; pp. 39–52. [Google Scholar]
- Renner, S.S.; Hausner, G. Siparunaceae, 1st ed.; New York Botanical Garden: Bronx, NY, USA, 2005; pp. 41–47. [Google Scholar]
- Renner, S.S.; Holm-Nielsen, L.B.; Balslev, H. Flowering Plants of Amazonian Ecuador: A Checklist, 1st ed.; AAU Reports 25; Aarhus Universitetsforlag: Aarhus, Denmark, 1991; pp. 57–89. [Google Scholar]
- Quijano-Abril, M.A.; Callejas-Posada, R.; Miranda-Esquivel, D.R. Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae). J. Biogeogr. 2006, 33, 1266–1278. [Google Scholar] [CrossRef]
- Céline, V.; Adriana, P.; Eric, D.; Joaquina, A.C.; Yannick, E.; Augusto, L.F.; Geneviève, B. Medicinal plants from the Yanesha (Peru): Evaluation of the leishmanicidal and antimalarial activity of selected extracts. J. Ethnopharmacol. 2009, 123, 413–422. [Google Scholar] [CrossRef]
- El-seedi, H.; Ghia, F.; Torssell, K.B.G. Cadinane sesquiterpenes from Siparuna macrotepala. Phytochemistry 1994, 35, 1495–1497. [Google Scholar] [CrossRef]
- Cicció, J.F. Essential oil from the leaves of Piper augustum from “Alberto M. Brenes” biological preserve, Costa Rica. J. Essent. Oil Res. 2005, 17, 251–253. [Google Scholar] [CrossRef]
- Swenson, J.T.; Nolan, L.L.; Roth, J.L. Antileishmanial properties of Amazonian plant extracts. In Proceedings of the International Symposium on Medicinal and Aromatic Plants, Amherst, MA, USA, 27–30 August 1995; Volume 426, pp. 201–210. [Google Scholar]
- Zhao, C.; Gong, X.; Chen, H.; Yang, Z.; Zhou, X. Analysis of volatile oil in Hedychium coronarium from Guizhou by SPME/GC/MS. China J. Tradit. Chin. Med. Pharm. 2010, 25, 1090–1092. [Google Scholar]
- Prakash, O.; Rajput, M.; Kumar, M.; Pant, A.K. Chemical Composition and Antibacterial Activity of Rhizome Oils from Hedychium coronarium Koenig and Hedychium spicatum Buch-Ham. J. Essent. Oil Bear. Plants 2010, 13, 250–259. [Google Scholar] [CrossRef]
- Dos Santos, B.C.; Barata, L.E.; Marques, F.A.; Baroni, A.C.; Karnos, B.A.; de Oliveira, P.R.; Guerrero, P.G., Jr. Composition of leaf and rhizome essential oils of Hedychium coronarium Koen. from Brazil. J. Essent. Oil. Res. 2010, 22, 305–306. [Google Scholar] [CrossRef]
- Joy, B.; Rajan, A.; Abraham, E. Antimicrobial activity and chemical composition of essential oil from Hedychium coronarium. Phytother. Res. 2007, 21, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.R.; Nasrin, M.; Saha, M.R. Evaluation of analgesic and neuropharmacological activities of methanolic rhizome extract of Hedychium coronarium. Int. J. Pharm. Sci. Res. 2011, 2, 979. [Google Scholar]
- Aziz, M.A.; Habib, M.R.; Karim, M.R. Antibacterial and cytotoxic activities of Hedychium coronarium J. Koenig. Res. J. Agric. Biol. Sci. 2009, 5, 969–972. [Google Scholar]
- Shrotriya, S.; Ali, M.S.; Saha, A.; Bachar, S.C.; Islam, M.S. Anti-inflammatory and analgesic effects of Hedychium coronarium Koenig. Pak. J. Pharm. Sci. 2007, 20, 47–51. [Google Scholar]
- Dixit, V.K.; Varma, K.C. Anthelmintic properties of essential oils from rhizomes of-Hedychium coronarium Koenig and Hedychium spicatum Koenig. Indian J. Pharm. 1975, 37, 143–144. [Google Scholar]
- Joshi, S.; Chanotiya, C.S.; Agarwal, G.; Prakash, O.; Pant, A.K.; Mathela, C.S. Terpenoid compositions, and antioxidant and antimicrobial properties of the rhizome essential oils of different Hedychium species. Chem. Biodivers. 2008, 5, 299–309. [Google Scholar] [CrossRef]
- Ho, J.C. Antimicrobial, mosquito larvicidal and antioxidant properties of the leaf and rhizome of Hedychium coronarium. J. Chin. Chem. Soc. 2011, 58, 563–567. [Google Scholar] [CrossRef]
- Suresh, G.; Reddy, P.P.; Babu, K.S.; Shaik, T.B.; Kalivendi, S.V. Two new cytotoxic labdane diterpenes from the rhizomes of Hedychium coronarium. Bioorg. Med. Chem. Lett. 2010, 20, 7544–7548. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Okazaki, Y.; Ninomiya, K.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Medicinal flowers. XXIV. Chemical structures and hepatoprotective effects of constituents from flowers of Hedychium coronarium. Chem. Pharm. Bull. 2008, 56, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Busines Media: Carol Stream, IL, USA, 2007. [Google Scholar]
- Røstelien, T.; Borg-Karlson, A.-K.; Fäldt, J.; Jacobsson, U.; Mustaparta, H. The Plant Sesquiterpene Germacrene D Specifically Activates a Major Type of Antennal Receptor Neuron of the Tobacco Budworm Moth Heliothis virescens. Chem. Senses 2000, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Noriega Rivera, P.F.; Guerrini, A.; Ankuash Tsamaraint, E. Composición química del aceite esencial de hojas de Siparuna schimpffii Diels (limoncillo). Rev. Cuba. Plantas Med. 2014, 19, 128–137. [Google Scholar]
- Bagheri, H.; Manap, M.Y.B.A.; Solati, Z. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef]
- Moraes, M.M.; da Silva, T.M.; da Silva, R.R.; Ramos, C.S.; da C.âmara, C.A. Circadian variation of essential oil from Piper marginatum Jacq. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2014, 13, 270–277. [Google Scholar]
- Costantin, M.B.; Sartorelli, P.; Limberger, R.; Henriques, A.T.; Steppe, M.; Ferreira, M.J.; Kato, M.J. Essential oils from Piper cernuum and Piper regnellii: Antimicrobial activities and analysis by GC/MS and 13C-NMR. Planta Med. 2001, 67, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.I.; Gupta, M.P. Potential of Panamanian aromatic flora as a source of novel essential oils. Biodivers. Int. J. 2018, 2, 405–413. [Google Scholar]
- Vuuren, S.V.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Frag. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Stoyanova, A.; Murgov, I.; Schmidt, E.; Geissler, M. Antimicrobial testing and gas chromatographic analysis of pure oxygenated monoterpenes 1,8-cineole, α-terpineol, terpinen-4-ol and camphor as well as target compounds in essential oils of pine (Pinus pinaster), rosemary (Rosmarinus officinalis), tea tree (Melaleuca alternifolia). Sci. Pharm. 2005, 73, 27–39. [Google Scholar]
- Noriega, P. Extracción, química, actividad biológica, control de calidad y potencial económico de los aceites esenciales. La Granja 2009, 10, 3–15. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.L.; Sacchetti, G.; Guerrini, A. Myrcia splendens (Sw.) DC. (syn. M. fallax (Rich.) DC.) (Myrtaceace) essential oil from Amazonian Ecuador: A chemical characterization and bioactive profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Rosen, R.T.; Ho, C.T. 2,2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J. Agric. Food Chem. 1999, 47, 2226–2228. [Google Scholar] [CrossRef]
- Rossi, D.; Guerrini, A.; Maietti, S.; Bruni, R.; Paganetto, G.; Poli, F.; Sacchetti, G. Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: A new functional food ingredient? Food Chem. 2011, 126, 837–848. [Google Scholar] [CrossRef]
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical Composition and Bio-efficacy of Essential Oils from Italian Aromatic Plants: Mentha suaveolens, Coridothymus capitatus, Origanum hirtum and Rosmarinus officinalis. Nat. Prod. Commun. 2016, 11, 1517–1520. [Google Scholar] [CrossRef]
- Popov, I.N.; Lewin, G. Photochemiluminescent detection of antiradical activity; IV: Testing of lipid-soluble antioxidants. J. Biochem. Bioph. Meth. 1996, 31, 1–8. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. [Google Scholar] [CrossRef]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Grandini, A.; Scalvenzi, L.; Rivera, P.F.N.; Andreotti, E.; Sacchetti, G. Biological and chemo-diverse characterization of Amazonian (Ecuador) Citrus petitgrains. J. Appl. Bot. Food Qual. 2014, 87, 108–116. [Google Scholar]
- Shahverdi, A.R.; Abdolpour, F.; Monsef-Esfahani, H.R.; Farsam, H. A TLC bioautographic assay for the detection of nitrofurantoin resistance reversal compound. J. Chromatogr. B 2007, 850, 528–530. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Molecules | AI Lit a | AI Exp b | RA c | ||||
|---|---|---|---|---|---|---|---|
| S. a | S. m | P. a | P. l | H. cd | |||
| α-pinene | 932 | 930 | 7.0 | 1.8 | 0.4 | 1.5 | 10.0 |
| camphene | 946 | 946 | 0.3 | 0.2 | - | - | 0.7 |
| sabinene e | 969 | 968 | - | - | - | - | 0.3 |
| β-pinene e | 974 | 975 | 2.1 | 0.5 | 0.5 | 1.7 | 30.0 |
| myrcene e | 990 | 991 | 0.1 | 0.1 | - | 0.1 | 0.5 |
| α-terpinene | 1017 | 1014 | - | - | - | - | 0.3 |
| p-cymene e | 1020 | 1021 | - | - | - | - | 1.2 |
| limonene e | 1024 | 1025 | 0.3 | 0.1 | 0.2 | 0.4 | 3.1 |
| β-phellandrene | 1025 | 1027 | - | - | - | - | 0.9 |
| 1,8-cineole e | 1026 | 1028 | 0.1 | - | - | 0.1 | 33.7 |
| (Z)-β-ocimene e | 1032 | 1035 | 0.3 | - | 0.2 | 0.4 | - |
| (E)-β-ocimene e | 1044 | 1050 | - | - | 1.8 | 3.5 | - |
| γ-terpinene | 1054 | 1052 | - | - | - | - | 1.2 |
| cis-sabinene hydrate | 1064 | 1067 | - | - | - | - | 0.1 |
| terpinolene | 1086 | 1081 | - | - | - | - | 0.3 |
| linalool e | 1095 | 1102 | - | - | - | - | 0.5 |
| perillene | 1102 | 1112 | - | 0.7 | - | ||
| endo-fenchol | 1114 | 1115 | - | - | - | - | 0.1 |
| cis-p-menth-2-en-1-ol | 1118 | 1121 | - | - | - | - | 0.1 |
| trans-pinocarveol | 1135 | 1135 | - | - | - | - | 0.1 |
| pinocarvone | 1160 | 1157 | - | - | - | - | 0.1 |
| borneol e | 1165 | 1166 | - | - | - | - | 1.1 |
| terpinen-4-ol e | 1174 | 1175 | - | - | - | - | 2.4 |
| α-terpineol e | 1186 | 1190 | 0.1 | - | - | - | 5.7 |
| 3,5-dimethoxytoluene | 1269 | 1264 | - | - | 0.5 | 1.4 | - |
| 2-undecanone | 1293 | 1293 | - | 0.3 | - | - | - |
| δ-elemene | 1335 | 1337 | 4.5 | - | 1.3 | 1.6 | - |
| α-cubebene | 1345 | 1351 | 1.7 | 1.8 | 0.3 | 0.3 | - |
| α-terpinyl acetate | 1346 | 1356 | - | - | - | - | 0.1 |
| cyclosativene | 1369 | 1370 | 0.2 | 0.1 | - | - | - |
| α-ylangene | 1373 | 1371 | 0.7 | 0.2 | 0.1 | - | - |
| α-copaene | 1374 | 1377 | 4.5 | 4.4 | 1.9 | 1.9 | - |
| β-bourbonene | 1387 | 1382 | 1.7 | 1.0 | 0.2 | 0.2 | - |
| β-cubebene | 1387 | 1388 | 0.3 | 1.9 | 0.2 | 0.4 | - |
| iso-longilofolene | 1389 | 1387 | 1.5 | - | - | - | - |
| β-elemenee | 1389 | 1391 | 2.3 | 1.5 | 5.8 | 5.1 | - |
| β-longipinene | 1400 | 1397 | - | - | 0.2 | 0.1 | - |
| E-β-caryophyllene e | 1417 | 1411 | 3.3 | 3.4 | 27.1 | 21.8 | 0.4 |
| β-copaene | 1430 | 1425 | 1.0 | 1.0 | 0.7 | 0.5 | - |
| β-gurjunene | 1431 | 1427 | 0.1 | - | - | - | - |
| γ-elemene | 1434 | 1425 | - | - | 0.1 | 0.1 | - |
| α-guaiene | 1437 | 1433 | 1.0 | 0.7 | - | - | - |
| aromandendrene | 1439 | 1430 | - | - | 0.5 | 0.4 | - |
| aristolene | 1450 | 1439 | 0.5 | - | - | - | - |
| cis-muurola-3,5-diene | 1448 | 1446 | 0.4 | 0.1 | 0.2 | 0.3 | - |
| trans-muurola-3,5-diene | 1451 | 1454 | - | 0.4 | - | - | - |
| α-humulene | 1454 | 1451 | 1.2 | 0.8 | 3.1 | 2.9 | 0.1 |
| allo-aromandendrene | 1458 | 1453 | 0.2 | 0.5 | 0.6 | 0.6 | - |
| dehydroaromadendrene | 1460 | 1460 | - | - | - | 0.1 | - |
| cis-cadina-1(6),4-diene | 1461 | 1459 | 0.5 | - | - | - | - |
| 9-epi- β-caryophyllene | 1464 | 1455 | 0.4 | - | 0.3 | 0.2 | - |
| cis-muurola-4(14),5-diene | 1465 | 1467 | - | 0.5 | - | - | - |
| γ-gurjunene | 1475 | 1471 | 0.7 | - | 0.7 | 0.5 | - |
| γ-muurolene | 1478 | 1475 | 2.2 | 0.5 | 1.9 | 1.3 | - |
| germacrene D f | 1484 | 1480 | 23.3 | 42.1 | 11.2 | 9.0 | - |
| β-selinene | 1489 | 1485 | 0.9 | 0.5 | 1.8 | 1.5 | 0.1 |
| drim-8(12)-ene | 1491 | 1484 | - | - | - | - | 0.1 |
| trans-muurola-4(14),5-diene | 1493 | 1484 | - | 1.1 | - | - | - |
| valencene | 1496 | 1487 | 0.7 | - | 0.6 | 0.4 | - |
| (Z,E)-α-farnesene | 1491 | 1491 | 3.2 | 2.7 | - | ||
| bicyclogermacrene | 1500 | 1492 | 7.8 | 11.8 | 5.2 | 4.0 | - |
| α-muurolene | 1500 | 1495 | 1.1 | 1.2 | 1.3 | 0.8 | - |
| β-himachalene | 1500 | 1498 | 1.2 | - | 0.4 | 0.2 | - |
| (E,E)-α-farnesene | 1505 | 1505 | - | 0.2 | 5.6 | 5.1 | - |
| germacrene A | 1508 | 1501 | 1.1 | - | 2.4 | 2.6 | - |
| γ-cadinene | 1513 | 1508 | 4.3 | 1.4 | 1.4 | 0.7 | - |
| cubebol | 1514 | 1510 | - | 0.3 | - | - | - |
| 7-epi-γ-selinene | 1522 | 1511 | - | - | - | 0.5 | - |
| δ-cadinene | 1522 | 1517 | 4.6 | 5.0 | 4.6 | 2.9 | - |
| cis-calamenene | 1528 | 1529 | - | - | 0.2 | 0.2 | - |
| zonarene | 1528 | 1530 | - | 0.2 | - | - | - |
| trans-cadina-1(2),4 diene | 1535 | 1531 | 0.3 | 0.4 | 0.3 | 0.2 | - |
| α-cadinene | 1537 | 1535 | 0.3 | 0.4 | 0.3 | 0.2 | - |
| α-calacorene | 1544 | 1540 | 0.3 | 0.1 | - | - | - |
| germacrene B | 1559 | 1557 | 1.3 | 1.7 | 1.2 | 1.2 | - |
| E-nerolidol e | 1561 | 1563 | - | - | 0.5 | 1.7 | - |
| spathulenol | 1577 | 1577 | 1.2 | 0.8 | 0.6 | 0.8 | - |
| caryophyllene oxide e | 1582 | 1581 | 0.2 | 0.1 | 0.8 | 3.8 | 0.2 |
| globulol | 1590 | 1585 | - | 0.5 | - | - | - |
| viridiflorol | 1592 | 1586 | 0.4 | 0.4 | 0.2 | 0.1 | - |
| carotol | 1594 | 1599 | - | - | - | 0.2 | - |
| guaiol | 1600 | 1597 | 0.4 | 0.4 | 0.1 | 0.2 | - |
| β-oplopenone | 1607 | 1609 | 0.5 | 0.1 | - | - | - |
| humulene 1,2-epoxide | 1608 | 1607 | - | - | - | 0.5 | - |
| 1,10-di-epi-cubenol | 1618 | 1617 | 0.1 | 0.2 | - | 0.1 | - |
| 10-epi-γ-eudesmol | 1622 | 1617 | - | - | - | 0.1 | - |
| 1-epi-cubenol | 1627 | 1630 | 0.4 | 0.6 | 0.4 | 0.5 | - |
| epi-α-cadinol | 1638 | 1646 | 0.7 | 0.7 | 0.5 | 0.4 | - |
| epi-α-muurolol | 1640 | 1648 | 0.5 | 0.7 | 0.7 | 0.7 | - |
| α-muurolol | 1644 | 1651 | 0.8 | 0.5 | 0.4 | 0.5 | - |
| α-cadinol | 1652 | 1660 | 1.2 | 1.5 | 0.8 | 0.7 | - |
| selin-11-en-4-α-ol | 1658 | 1660 | - | - | 0.6 | 0.7 | - |
| intermedeol | 1665 | 1668 | - | - | 0.3 | 0.5 | - |
| khusinol | 1679 | 1689 | 0.4 | - | - | - | - |
| eudesma-4(15),7-dien-1-β-ol | 1687 | 1696 | 0.2 | - | - | - | - |
| cyclocolorenone | 1759 | 1761 | - | - | 0.1 | 0.1 | - |
| Total identified (%) | 93.1 | 94.7 | 94.5 | 91.9 | 93.4 | ||
| Essential Oils and Pure Molecules | IC50 mg/mL | |
|---|---|---|
| DPPH | ABTS | |
| S. aspera | 20.70 ± 0.80 | 1.12 ± 0.04 |
| S. macrotepala | 29.37 ± 1.15 | 0.80 ± 0.03 |
| P. augustum | 6.17 ± 0.33 | 2.16 ± 0.20 |
| P. leticianum | 4.26 ± 0.11 | 2.65 ± 0.25 |
| H. coronarium | 9.04 ± 0.55 | 2.87 ± 0.17 |
| T. vulgaris | 0.71 ± 0.02 | 0.055 ± 0.001 |
| E-β-caryophyllene | 80.1 ± 1.40 | 15.1 ± 1.16 |
| β-pinene | 149.8 ± 5.66 | 142.0 ± 9.07 |
| 1,8-cineole | 440.8 ± 10.18 | 174.1 ± 7.44 |
| germacrene D | 2.1 ± 0.02 | 1.19 ± 0.02 |
| Essential Oils | μmol of Trolox/mL (p ≤ 0.05) |
|---|---|
| S. aspera | 4.72 ± 0.08 |
| S. macrotepala | 5.43 ± 0.15 |
| P. augustum | 1.07 ± 0.03 |
| P. leticianum | 1.35 ± 0.04 |
| H. coronarium | 9.04 ± 0.05 |
| T. vulgaris | 283.33 ± 8.57 |
| Microorganism | S. aspera MIC (mg/mL) | S. macrotepala MIC (mg/mL) | P. augustum MIC (mg/mL) | P. leticianum MIC (mg/mL) | H coronarium MIC (mg/mL) | T. vulgaris MIC (mg/mL) | |
|---|---|---|---|---|---|---|---|
| Gram + bacteria | EF | 9.3 | 9.0 | 9.1 | 9.1 | 9.0 | 1.8 |
| LIST | 9.3 | 9.3 | 18.2 | 18.1 | 0.45 | 0.9 | |
| MLU | 4.6 | 18.6 | 18.2 | 91.1 | 9.0 | 1.8 | |
| SAU | 46.0 | 46.5 | 91.0 | 18.1 | 9.0 | 1.8 | |
| SE | 18.6 | 18.6 | 18.2 | 18.1 | 4.5 | 0.9 | |
| SMU | 1.9 | 0.9 | 0.18 | -0.18 | 0.18 | 0.18 | |
| Gram − bacteria | EC | 464 | 465 | 454 | 453 | 89.5 | 4.6 |
| KOX | 18.6 | 46.5 | 45.4 | 45.3 | 0.9 | 0.9 | |
| PVU | 18.6 | 46.5 | 45.4 | 18.1 | 9.0 | 0.9 | |
| PA | 464 | 93.0 | 91.0 | 9.,6 | 89.5 | 9.2 | |
| Yeasts | SC | 92.9 | 465.0 | 18.2 | 18.1 | 89.5 | 1.8 |
| CAND | 46.0 | 93.0 | 91.0 | 45.3 | 17.9 | 1.8 | |
| MF | 18.6 | 46.5 | 1.8 | 18.1 | 4.5 | 0.18 | |
| Species | Site Collection | Geographical Coordinates |
|---|---|---|
| S. aspera | San Luis del Upano parish, Morona Santiago province. | Latitude: S 2°28′43″ Length: W 78°8′59″ Altitude: 820 msm |
| S. macrotepala | Shakaim Biological Station, Chiguaza parish, Morona Santiago province. | Latitude: S 02°03′52.2″, Length: W 77°52′32.5″ Altitude: 1200 msm |
| P. augustum | Shakaim Biological Station, Chiguaza parish, Morona Santiago province. | Latitude: S 02°03′52.2″, Length: W 77°52′32.5″ Altitude: 1200 msm |
| P. leticianum | Shakaim Biological Station, Chiguaza parish, Morona Santiago province. | Latitude: S 02°03′52.2″, Length: W 77°52′32.5″ Altitude: 1200 msm |
| H. coronarium | Macas, Morona Santiago province | Latitude: S 2°10′ Length: W 78°0′ Altitude: 1080 msm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules 2019, 24, 1637. https://doi.org/10.3390/molecules24081637
Noriega P, Guerrini A, Sacchetti G, Grandini A, Ankuash E, Manfredini S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules. 2019; 24(8):1637. https://doi.org/10.3390/molecules24081637
Chicago/Turabian StyleNoriega, Paco, Alessandra Guerrini, Gianni Sacchetti, Alessandro Grandini, Edwin Ankuash, and Stefano Manfredini. 2019. "Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest" Molecules 24, no. 8: 1637. https://doi.org/10.3390/molecules24081637
APA StyleNoriega, P., Guerrini, A., Sacchetti, G., Grandini, A., Ankuash, E., & Manfredini, S. (2019). Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules, 24(8), 1637. https://doi.org/10.3390/molecules24081637