Packing Rearrangements in 4-Hydroxycyanobenzene Under Pressure
Abstract
:1. Introduction
2. Results
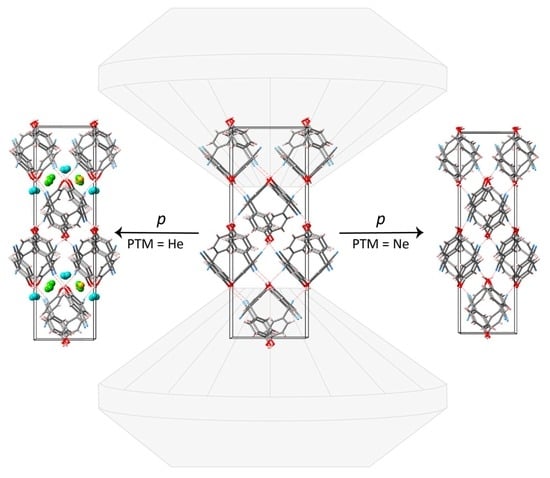
2.1. Compression in Helium
2.2. Compression in Neon
3. Discussion
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Sun, L.; Wang, C.; Yang, F.; Ren, X.; Zhang, X.; Dong, H.; Hu, W. Organic crystalline materials in flexible electronics. Chem. Soc. Rev. 2019, 48, 1492–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Soc. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2011, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Osaki, K. p-Cyanophenol. Acta Cryst. B 1977, 33, 607–609. [Google Scholar] [CrossRef]
- Fraboni, B.; DiPietro, R.; Castaldini, A.; Cavallini, A.; Fraleoni-Morgera, A.; Setti, L.; Mencarelli, I.; Femoni, C. Anisotropic charge transport in organic single crystals based on dipolar molecules. Org. Electron. 2008, 9, 974–978. [Google Scholar] [CrossRef]
- Fraboni, B.; Femoni, C.; Mencarelli, I.; Setti, L.; Di Pietro, R.; Cavallini, A.; Fraleoni-Morgera, A. Solution-Grown, Macroscopic Organic Single Crystals Exhibiting Three-Dimensional Anisotropic Charge-Transport Properties. Adv. Mater. 2009, 21, 1835–1839. [Google Scholar] [CrossRef]
- Fraboni, B.; Fraleoni-Morgera, A.; Cavallini, A. Three-dimensional anisotropic density of states distribution and intrinsic-like mobility in organic single crystals. Org. Electron. 2010, 11, 10–15. [Google Scholar] [CrossRef]
- Fraleoni-Morgera, A.; Tessarolo, M.; Perucchi, A.; Baldassarre, L.; Lupi, S.; Fraboni, B. Polarized Infrared Studies on Charge Transport in 4-Hydroxycyanobenzene Single Crystals. J. Phys. Chem. C 2012, 116, 2563–2569. [Google Scholar] [CrossRef]
- Fraboni, B.; Ciavatti, A.; Merlo, F.; Pasquini, L.; Cavallini, A.; Quaranta, A.; Bonfiglio, A.; Fraleoni-Morgera, A. Organic Semiconducting Single Crystals as Next Generation of Low-Cost, Room-Temperature Electrical X-ray Detectors. Adv. Mater. 2012, 24, 2289–2293. [Google Scholar] [CrossRef]
- Ciavatti, A.; Capria, E.; Fraleoni-Morgera, A.; Tromba, G.; Dreossi, D.; Sellin, P.J.; Cosseddu, P.; Bonfiglio, A.; Fraboni, B. Toward Low-Voltage and Bendable X-Ray Direct Detectors Based on Organic Semiconducting Single Crystals. Adv. Mater. 2015, 27, 7213–7220. [Google Scholar] [CrossRef] [Green Version]
- Mohanraj, J.; Capria, E.; Benevoli, L.; Perucchi, A.; Demitri, N.; Fraleoni-Morgera, A. XRD- and infrared-probed anisotropic thermal expansion properties of an organic semiconducting single crystal. Phys. Chem. Chem. Phys. 2018, 20, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Cossaro, A.; Capria, E.; Benevoli, L.; Coreno, M.; De Simone, M.; Prince, K.C.; Kladnik, G.; Cvetko, D.; Fraboni, B.; et al. Intermolecular Hydrogen Bonding and Molecular Orbital Distortion in 4-Hydroxycyanobenzene Investigated by X-ray Spectroscopy. J. Phys. Chem. C 2015, 119, 121–129. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π − π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, Y. High-Pressure-Induced Phase Transition in 2,5-Diketopiperazine: The Anisotropic Compression of N–H⋯O Hydrogen-Bonded Tapes. J. Phys. Chem. C 2018, 122, 11747–11753. [Google Scholar] [CrossRef]
- Cai, W.; Katrusiak, A. Pressure effects on H-ordering in hydrogen bonds and interactions in benzoic acid. CrystEngComm 2012, 14, 4420–4424. [Google Scholar] [CrossRef]
- Fanetti, S.; Citroni, M.; Dziubek, K.; Nobrega, M.M.; Bini, R. The role of H-bond in the high-pressure chemistry of model molecules. J. Phys. Condens. Matter 2018, 30, 094001. [Google Scholar] [CrossRef] [Green Version]
- Kang, L.; Wang, K.; Li, X.; Zou, B. High Pressure Structural Investigation of Benzoic Acid: Raman Spectroscopy and X-ray Diffraction. J. Phys. Chem. C 2016, 120, 14758–14766. [Google Scholar] [CrossRef]
- Orgzall, I.; Emmerling, F.; Schulz, B.; Franco, O. High-pressure studies on molecular crystals—Relations between structure and high-pressure behavior. J. Phys. Condens. Matter 2008, 20, 295206. [Google Scholar] [CrossRef]
- Stavrou, E.; Riad Manaa, M.; Zaug, J.M.; Kuo, I.F.W.; Pagoria, P.F.; Kalkan, B.; Crowhurst, J.C.; Armstrong, M.R. The high pressure structure and equation of state of 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) up to 20 GPa: X-ray diffraction measurements and first principles molecular dynamics simulations. J. Chem. Phys. 2015, 143, 144506. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, L.; Zheng, H.; Li, K.; Andrzejewski, M.; Hattori, T.; Sano-Furukawa, A.; Katrusiak, A.; Meng, Y.; Liao, F.; et al. Phase Transitions and Polymerization of C6H6–C6F6 Cocrystal under Extreme Conditions. J. Phys. Chem. C 2016, 120, 29510–29519. [Google Scholar] [CrossRef]
- Casati, N.; Kleppe, A.; Jephcoat, A.P.; Macchi, P. Putting pressure on aromaticity along with in situ experimental electron density of a molecular crystal. Nat. Commun. 2016, 7, 10901. [Google Scholar] [CrossRef] [Green Version]
- Cliffe, M.J.; Goodwin, A.L. PASCal: A principal-axis strain calculator for thermal expansion and compressibility determination. J. Appl. Cryst. 2012, 45, 1321–1329. [Google Scholar] [CrossRef]
- Laniel, D.; Sebastiao, E.; Cook, C.; Murugesu, M.; Hu, A.; Zhang, F.; Desgreniers, S. Dense nitrogen-rich energetic materials: A study of 5,5′-bis(1H-tetrazolyl)amine. J. Chem. Phys. 2014, 140, 184701. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.; Orgzall, I.; Reck, G.; Stockhause, S.; Schulz, B. Structure and high-pressure behavior of 2,5-di-(4-aminophenyl)-1,3,4-oxadiazole. J. Phys. Chem. Solids 2005, 66, 994–1003. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Ciabini, L.; Gorelli, F.A.; Santoro, M.; Bini, R.; Schettino, V.; Mezouar, M. High-pressure and high-temperature equation of state and phase diagram of solid benzene. Phys. Rev. B 2005, 72, 094108. [Google Scholar] [CrossRef]
- Nobrega, M.M.; Temperini, M.L.A.; Bini, R. Probing the Chemical Stability of Aniline under High Pressure. J. Phys. Chem. C 2017, 121, 7495–7501. [Google Scholar] [CrossRef]
- Citroni, M.; Fanetti, S.; Bazzicalupi, C.; Dziubek, K.; Pagliai, M.; Nobrega, M.M.; Mezouar, M.; Bini, R. Structural and electronic competing mechanisms in the formation of amorphous carbon nitride by compressing s-triazine. J. Phys. Chem. C 2015, 119, 28560–28569. [Google Scholar] [CrossRef]
- Badenhoop, J.K.; Weinhold, F. Natural steric analysis: Ab initio van der Waals radii of atoms and ions. J. Chem. Phys. 1997, 107, 5422–5432. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.C.; Munsch, P.; Marchand, G.L. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Guńka, P.A.; Dziubek, K.F.; Gładysiak, A.; Dranka, M.; Piechota, J.; Hanfland, M.; Katrusiak, A.; Zachara, J. Compressed arsenolite As4O6 and its helium clathrate As4O6·2He. Cryst. Growth Des. 2015, 15, 3740–3745. [Google Scholar] [CrossRef]
- Guńka, P.A.; Hapka, M.; Hanfland, M.; Dranka, M.; Chałasiński, G.; Zachara, J. How and Why Does Helium Permeate Nonporous Arsenolite Under High Pressure? ChemPhysChem 2018, 19, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sans, J.A.; Manjón, F.J.; Popescu, C.; Cuenca-Gotor, V.P.; Gomis, O.; Muñoz, A.; Rodríguez-Hernández, P.; Contreras-García, J.; Pellicer-Porres, J.; Pereira, A.L.; et al. Ordered helium trapping and bonding in compressed arsenolite: Synthesis of As4O6·2He. Phys. Rev. B 2016, 93, 054102. [Google Scholar] [CrossRef]
- Hester, B.R.; dos Santos, A.M.; Molaison, J.J.; Hancock, J.C.; Wilkinson, A.P. Synthesis of Defect Perovskites (He2−x□x)(CaZr)F6 by Inserting Helium into the Negative Thermal Expansion Material CaZrF6. J. Am. Chem. Soc. 2017, 139, 13284–13287. [Google Scholar] [CrossRef] [PubMed]
- Collings, I.E.; Bykov, M.; Bykova, E.; Hanfland, M.; van Smaalen, S.; Dubrovinsky, L.; Dubrovinskaia, N. Disorder–order transitions in the perovskite metal–organic frameworks [(CH3)2NH2][M(HCOO)3] at high pressure. CrystEngComm 2018, 20, 3512–3521. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.38.46; Rigaku Corporation: Oxford, UK, 2018. [Google Scholar]
- Sheldrick, G. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]









| PTM | P Range (GPa) | |||
|---|---|---|---|---|
| He | 0–2.1 | 6.0(6) | 2525.3(3) | 10.0(14) |
| He | 10.4–25.1 | 11.1(3) | 2520(15) | 6.65(10) |
| Ne | 0–4.0 | 5.5(2) | 2516.7(10) | 11.0(4) |
| Ne | 6-14 | 9.7(2) | 2377(10) | 6.78(10) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collings, I.E.; Hanfland, M. Packing Rearrangements in 4-Hydroxycyanobenzene Under Pressure. Molecules 2019, 24, 1759. https://doi.org/10.3390/molecules24091759
Collings IE, Hanfland M. Packing Rearrangements in 4-Hydroxycyanobenzene Under Pressure. Molecules. 2019; 24(9):1759. https://doi.org/10.3390/molecules24091759
Chicago/Turabian StyleCollings, Ines E., and Michael Hanfland. 2019. "Packing Rearrangements in 4-Hydroxycyanobenzene Under Pressure" Molecules 24, no. 9: 1759. https://doi.org/10.3390/molecules24091759
APA StyleCollings, I. E., & Hanfland, M. (2019). Packing Rearrangements in 4-Hydroxycyanobenzene Under Pressure. Molecules, 24(9), 1759. https://doi.org/10.3390/molecules24091759