Selective Synthesis and Photoluminescence Study of Pyrazolopyridopyridazine Diones and N-Aminopyrazolopyrrolopyridine Diones
Abstract
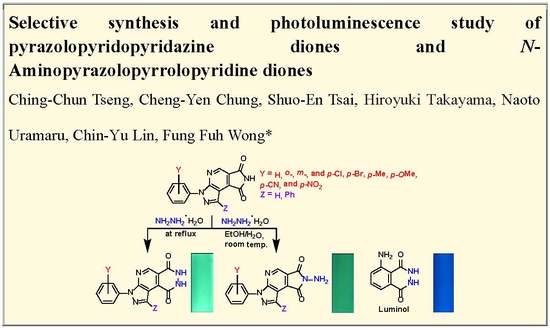
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Standard Procedure for Synthesis of Pyrazolopyridopyridazine Diones 6a–j
3.3. Standard Procedure for Synthesis of N-Aminopyrazolopyrrolopyridine Diones (7a–i)
3.4. Determination of the Fluorescence Quantum Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boivin, D.B.; Tremblay, G.M.; James, F.O. Working on Atypical Schedules. Sleep Med. 2007, 8, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, R.; Silva, G.M.; Zapata-Sudo, G.; Raimundo, J.M.; Sudo, R.T.; Barreiro, E.J.; Fraga, C.A.M. Design, Synthesis, and Pharmacological Evaluation of New Neuroactive Pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine Derivatives with in vivo Hypnotic and Analgesic Profile. Bioorg. Med. Chem. 2006, 14, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Junior, N.M.; Mendes, T.C.F.; Leal, D.M.; Correa, C.M.N.; Sudo, R.T.; Zapata-Sudo, G.; Barreiro, E.J.; Fraga, C.A.M. Microwave-assisted Synthesis and Structure–activity Relationships of Neuroactive pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine Derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Macor, J.E.; Chung, H.-J.; Humora, M.; Katipally, K.; Wang, Y.; Kim, S. Fused Pyridopyridazine Inhibitors of cGMP Phosphodiesterase. U.S. Patent No. 6,316,438, 13 November 2001. [Google Scholar]
- Yu, G.; Mason, H.; Wu, X.; Wang, J.; Chong, S.; Beyer, B.; Henwood, A.; Pongrac, R.; Seliger, L.; He, B.; et al. Substituted Pyrazolopyridopyridazines as Orally Bioavailable Potent and Selective PDE5 Inhibitors: Potential Agents for Treatment of Erectile Dysfunction. J. Med. Chem. 2003, 46, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, H.; Trush, M.A. Detection of Mitochondria-derived Reactive Oxygen Species Production by the Chemilumigenic Probes Lucigenin and Luminol. Biochim. Biophys. Acta 1999, 1428, 1–12. [Google Scholar] [CrossRef]
- García-Campaña, A.M.; Baeyens, W.R.G. Chemiluminescence in Chemical Analysis; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Roda, A. Chemiluminescence and Bioluminescence: Past, Present, and Future; RSC: Cambridge, UK, 2011. [Google Scholar]
- Barni, F.; Lewis, S.W.; Berti, A.; Miskelly, G.M.; Lago, G. Forensic Application of the Luminol Reaction as a Presumptive Test for Latent Blood Detection. Talanta 2007, 72, 896–913. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, H.; Zhou, Y.; Wang, L.; Fu, Z. A Novel Disposable Immunosensor based on Quenching of Electrochemiluminescence Emission of Ru(bpy)32+ by Amorphous Carbon Nanoparticles. Sens. Actuators B 2015, 209, 744–750. [Google Scholar] [CrossRef]
- Yoshida, H.; Ureshino, K.; Ishida, J.; Nohta, H.; Yamaguchi, M. Chemiluminescent Properties of some Luminol related Compounds (II). Dyes Pigm. 1999, 41, 177–182. [Google Scholar] [CrossRef]
- Gu, W.; Deng, X.; Gu, X.; Jia, X.; Lou, B.; Zhang, X.; Li, J.; Wang, E. Stabilized, Superparamagnetic Functionalized Graphene/Fe3O4@Au Nanocomposites for a Magnetically-controlled Solid-state Electrochemiluminescence. Biosensing Application. Anal. Chem. 2015, 87, 1876–1881. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Hao, N.; Feng, Q.-M.; Shi, H.-W.; Xu, J.-J.; Chen, H.-Y. A Ratiometric Electrochemiluminescence Detection for Cancer Cells using g-C3N4 Nanosheets and Ag–PAMAM–luminol nanocomposites. Biosens. Bioelectron. 2016, 77, 76–82. [Google Scholar] [CrossRef]
- Chan, C.M. An improved Synthesis of a Chemiluminescent Cyclic Hydrazide: N-(7-Aminobutyl)-N-ethyl-naphthalene-1,2-dicarboxylic hydrazide. Synth. Commun. 1989, 19, 1981–1985. [Google Scholar] [CrossRef]
- Ishida, J.; Yamaguchi, M.; Nakahara, T.; Nakamura, M. 4,5-Diaminophthalhy drazide as a highly Sensitive Chemiluminescence Reagent for α-Keto Acids in Liquid Chromatography. Anal. Chim. Acta 1990, 231, 1–6. [Google Scholar] [CrossRef]
- Sasamoto, K.; Ohkura, Y. A New Chemiluminogenic Substrate for N-Acetyl-β-D-glucosaminidase, 4′-(6′-Diethylaminobenzofuranyl)phthalylhydrazido-N-acetyl-β-D-glucosaminide. Chem. Pharm. Bull. 1991, 39, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Ishida, J.; Takada, M.; Yakabe, T.; Yamaguchi, M. Chemiluminescent Properties of some Luminol related Compounds. Dyes Pigm. 1995, 27, 1–7. [Google Scholar] [CrossRef]
- Ishida, J.; Takada, M.; Hara, S.; Sasamoto, K.; Kina, K.; Yamaguchi, M. Development of a Novel Chemiluminescent Probe, 4-(5′,6′-dimethoxybenzothiazolyl)phthalhydrazide. Anal. Chim. Acta 1995, 309, 211–219. [Google Scholar] [CrossRef]
- Yoshida, H.; Nakao, R.; Nohta, H.; Yamaguchi, M. Chemiluminescent Properties of some Luminol-related compounds—Part 3. Dyes Pigm. 2000, 47, 239–245. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Antonov, L. Tautomerism: Methods and Theories, 1st ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Ali Ahmed, H.E.; Abdel-Salam, H.A.; Shaker, M.A. Synthesis, Characterization, Molecular Modeling, and Potential Antimicrobial and Anticancer Activities of Novel 2-Aminoisoindoline-1,3-dione derivatives. Bioorg. Chem. 2016, 66, 1–11. [Google Scholar] [CrossRef]
- Conchon, E.; Anizon, F.; Aboab, B.; Golsteyn, R.M.; Léonce, S.; Pfeiffer, B.; Prudhomme, M. Synthesis, in Vitro Antiproliferative Activities, and Chk1 Inhibitory Properties of Pyrrolo[3,4-a]carbazole-1,3-diones, Pyrrolo[3,4-c]carbazole-1,3-diones, and 2-Aminopyridazino[3,4-a]pyrrolo[3,4-c]carbazole-1,3,4,7-tetraone. Eur. J. Med. Chem. 2008, 43, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.-C.; Yen, W.-P.; Tsai, A.-E.; Hu, Y.-T.; Takayama, H.; Kuo, Y.-H.; Wong, F.F. ZnCl2-Catalyzed Aza-Diels–Alder Reaction for the Synthesis of 1H-Pyrazolo[3,4-b]pyridine-4,5-dicarboxylate Derivatives. Eur. J. Org. Chem. 2018, 2018, 1567–1571. [Google Scholar]
- Deshmukh, M.S.; Sekar, N. Chemiluminescence properties of luminol related quinoxaline analogs: Experimental and DFT based approach to photophysical properties. Dyes Pigm. 2015, 117, 49–60. [Google Scholar] [CrossRef]
- Deshmukh, M.S.; Sekar, N. Chemiluminescence Properties of Luminol related o-Hydroxybenzimidazole analogues: Experimental and DFT based Approach to Photophysical Properties. Dyes Pigm. 2015, 113, 189–199. [Google Scholar] [CrossRef]
- Peryasami, G.; Martelo, L.; Baleizã, C.; Berberan-Santos, M.N. Strong Green Chemiluminescence from Naphthalene analogues of Luminol. New J. Chem. 2014, 38, 2258–2261. [Google Scholar] [CrossRef]
- Spurlin, S.R.; Cooper, M.M. A Chemiluminescent Precolumn Labelling Reagent for High-Performance Liquid Chromatography of Amino Acids. Anal. Lett. 1986, 19, 2277–2283. [Google Scholar] [CrossRef]
- Yen, W.-P.; Liu, P.-L.; Uramaru, N.; Wong, F.F. Indium(III) chloride/silica gel catalyzed synthesis of pyrazolo[3,4-b]pyrrolo[3,4-d]pyridines. Tetrahedron 2015, 71, 8798–8803. [Google Scholar] [CrossRef]
- Neumann, H.; Klaus, S.; Klawona, M.; Strűbina, D.; Hűbner, S.; Gördes, D.; von Wangelin, A.J.; Lalk, M.; Beller, M. A New Efficient Synthesis of Substituted Luminols using Multicomponent Reactions. Z. Naturforsch. 2004, 59b, 431–438. [Google Scholar] [CrossRef]
- Flitsch, W.; Krämer, U.; Zimmerman, H. Cyclische Verbindungen mit Heterobrückenatomen, V. Zur Chemie der 1-Amino-pyrrole. Chem. Ber. 1969, 102, 3268–3276. [Google Scholar] [CrossRef]
- Dey, S.K.; Lightner, D.A. 1,1′-Bipyrroles: Synthesis and Stereochemistry. J. Org. Chem. 2007, 72, 9395–9397. [Google Scholar] [CrossRef] [Green Version]
- Gompper, R.; Sobotta, R. Neue Elektronenreiche Butadiene. Tetrahedron Lett. 1979, 11, 921–924. [Google Scholar] [CrossRef]
- Fang, J.M.; Yang, C.C.; Wang, Y.W. Use of α-Anilino Dienenitriles as Nucleophiles in Cycloadditions. J. Org. Chem. 1989, 54, 477–481. [Google Scholar] [CrossRef]
- Tyagi, P.; Venkateswararao, A.; Thomas, K.R.J. Solution Processable Indoloquinoxaline derivatives containing Bulky Polyaromatic Hydrocarbons: Synthesis, Optical Spectra, and Electroluminescence. J. Org. Chem. 2011, 76, 4571–4581. [Google Scholar] [CrossRef]
- Martelo, L.; Periyasami, G.; Fedorov, A.A.; Baleizão, C.; Berberan-Santos, M.N. Chemiluminescence of Naphthalene analogues of Luminol in Solution and Micellar Media. Dyes Pigm. 2019, 168, 341–346. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Dutkiewlcz, M. Classification of Organic Solvents based on Correlation between Dielectric β Parameter and Empirical Solvent Polarity Parameter ENT. J. Chem. Soc. Faraday Trans. 1990, 86, 2237–2241. [Google Scholar] [CrossRef]
- Behera, S.K.; Karak, A.; Krishnamoorthy, G. Photophysics of 2-(4′-Amino-2′-hydroxyphenyl)-1H-imidazo-[4,5-c]pyridine and its analogues: Intramolecular Proton Transfer Versus Intramolecular Charge Transfer. J. Phys. Chem. B 2015, 119, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Murkherjee, A.; Sadhuragiri, G.; Elumalai, P.; Sathiyendiran, M.; Kumar, M.; Mandal, B.B.; Krishnamoorthy, G. Aggregation induced enhanced and Exclusively Highly Stokes Shifted Emission from an Excited State Intramolecular Proton Transfer Exhibiting Molecule. Faraday Discuss. 2017, 196, 71–90. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Wu, P.-J.; Peng, C.-Y.; Shen, J.-Y.; Tsai, C.-C.; Hu, W.-P.; Chou, P.-T. A study of the Competitive Multiple Hydrogen Bonding Effect and its associated Excited-state Proton Transfer Tautomerism. Phys. Chem. Chem. Phys. 2017, 19, 28641–28646. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, Y.; Wang, R.; Wu, H.; Zhang, Y. Acid-induced Shift of Intramolecular Hydrogen Bonding Responsible for Excited-state Intramolecular Proton Transfer. Chem. Asian J. 2018, 13, 1735–1743. [Google Scholar] [CrossRef]
- Tang, Z.; Lu, M.; Liu, K.; Zhao, Y.; Qi, Y.; Wang, Y.; Zhang, P.; Zhou, P. Solvation Effect on the ESIPT Mechanism of 2-(4′-Amino-2′-hydroxyphenyl)-1H-imidazo-[4,5-c]pyridine. J. Photochem. Photobiol. A 2018, 367, 261–269. [Google Scholar] [CrossRef]
- Satapathy, A.K.; Behera, S.K.; Yadav, A.; Laxmi Mahour, L.N.; Yelamaggad, C.V.; Sandhya, K.L.; Sahoo, B. Tuning the Fluorescence Behavior of Liquid Crystal Molecules containing Schiff-base: Effect of Solvent Polarity. J. Lumin. 2019, 210, 371–375. [Google Scholar] [CrossRef]
- Skripnikova, T.A.; Lysova, S.S.; Zevatskii, Y.E.; Myznikov, L.V.; Vorona, S.V.; Artamonva, T.V. Physico-chemical Properties of Isomeric Forms of Luminol in Aqueous Solutions. J. Mol. Struct. 2018, 1154, 59–63. [Google Scholar] [CrossRef]
- Deepa, S.; Reddy, S.R.; Rajendrakumar, K. Green Chemiluminescence of Highly Fluorescent Symmetrical Azo-based Luminol derivative. Orient. J. Chem. 2018, 34, 894–905. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Kiyama, M.; Iwano, S.; Otsuka, S.; Lu, S.W.; Obata, R.; Miyawaki, A.; Hirano, T.; Maki, S.A. Quantum Yield Improvement of Red-light-emitting Firefly Luciferin Analogues for in vivo Bioluminescence Imaging. Tetrahedron 2018, 74, 652–660. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |










| Entry | S.M. | X | Y | Z | Reaction Time | Products | Yields (%) |
|---|---|---|---|---|---|---|---|
| 1 | 11a | NH | Ph | Ph | 5 | 6a | 84 |
| 2 | 12a | NMe | Ph | Ph | 5 | 6a | 32 |
| 3 | 13a | O | Ph | Ph | 5 | 6a | 18 |
| 4 | 11b | NH | o-Cl-Ph | Ph | 5 | 6b | 74 |
| 5 | 12b | NMe | o-Cl-Ph | Ph | 5 | 6b | 24 |
| 6 | 13b | O | o-Cl-Ph | Ph | 5 | 6b | 13 |
| 7 | 11c | NH | m-Cl-Ph | Ph | 5 | 6c | 71 |
| 8 | 12c | NMe | m-Cl-Ph | Ph | 5 | 6c | 38 |
| 9 | 13c | O | m-Cl-Ph | Ph | 5 | 6c | 11 |
| 10 | 11d | NH | p-Cl-Ph | Ph | 5 | 6d | 81 |
| 11 | 11e | NH | p-Br-Ph | Ph | 5 | 6e | 77 |
| 12 | 11f | NH | p-Me-Ph | Ph | 5 | 6f | 84 |
| 13 | 11g | NH | p-OMe-Ph | Ph | 5 | 6g | 81 |
| 14 | 11h | NH | p-CN-Ph | Ph | 5 | 6h | 73 |
| 15 | 11i | NH | p-NO2-Ph | Ph | 5 | 6i | 69 |
| 16 | 11j | NH | m-Cl-Ph | H | 5 | 6j | 71 |
| Compound | Solvent | λmax/nm of UV-Vis | λmax/nm of PL |
|---|---|---|---|
| 6a | Toluene | - 1,366 | 469 |
| 6a | THF | 271,358 | 471 |
| 6a | Ethyl acetate | 268, 356 | 473 |
| 6a | CH2Cl2 | 271, 350 | 483 |
| 6a | MeCN | 268, 351 | 488 |
| 6a | Acetone | - 1, 353 | 477 |
| 6a | DMSO | 264, 338 | 486 |
| 7a | Toluene | 286, 1 ,348 | 452 |
| 7a | THF | 264, 344 | 454 |
| 7a | Ethyl acetate | 262, 343 | 459 |
| 7a | CH2Cl2 | 264, 344 | 471 |
| 7a | MeCN | 261, 329 | 425, 461 |
| 7a | Acetone | - 1, 338 | 452 |
| 7a | DMSO | 264, 335 | 429, 478 |
| Luminol (1) | DMSO | 350 | 392 |
| Compound | Solvent | λfl 1/nm | Φf 2 |
|---|---|---|---|
| 6a | CH2Cl2 | 481 | 0.056 |
| 6c | CH2Cl2 | 472 | 0.067 |
| 6j | CH2Cl2 | 450 | 0.140 |
| 6j | THF | 435 | 0.218 |
| 6j | Toluene | 438 | 0.209 |
| 6j | Acetone | 437 | 0.083 |
| 6j | Ethyl acetate | 437 | 0.049 |
| Luminol (1) | CH2Cl2 | 399 | 0.175 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-C.; Chung, C.-Y.; Tsai, S.-E.; Takayama, H.; Uramaru, N.; Lin, C.-Y.; Wong, F.F. Selective Synthesis and Photoluminescence Study of Pyrazolopyridopyridazine Diones and N-Aminopyrazolopyrrolopyridine Diones. Molecules 2020, 25, 2409. https://doi.org/10.3390/molecules25102409
Tseng C-C, Chung C-Y, Tsai S-E, Takayama H, Uramaru N, Lin C-Y, Wong FF. Selective Synthesis and Photoluminescence Study of Pyrazolopyridopyridazine Diones and N-Aminopyrazolopyrrolopyridine Diones. Molecules. 2020; 25(10):2409. https://doi.org/10.3390/molecules25102409
Chicago/Turabian StyleTseng, Ching-Chun, Cheng-Yen Chung, Shuo-En Tsai, Hiroyuki Takayama, Naoto Uramaru, Chin-Yu Lin, and Fung Fuh Wong. 2020. "Selective Synthesis and Photoluminescence Study of Pyrazolopyridopyridazine Diones and N-Aminopyrazolopyrrolopyridine Diones" Molecules 25, no. 10: 2409. https://doi.org/10.3390/molecules25102409
APA StyleTseng, C. -C., Chung, C. -Y., Tsai, S. -E., Takayama, H., Uramaru, N., Lin, C. -Y., & Wong, F. F. (2020). Selective Synthesis and Photoluminescence Study of Pyrazolopyridopyridazine Diones and N-Aminopyrazolopyrrolopyridine Diones. Molecules, 25(10), 2409. https://doi.org/10.3390/molecules25102409