Synthesis, Crystal Structures and Luminescence Properties of Three New Cadmium 3D Coordination Polymers
Abstract
:1. Introduction
2. Results and Discussion
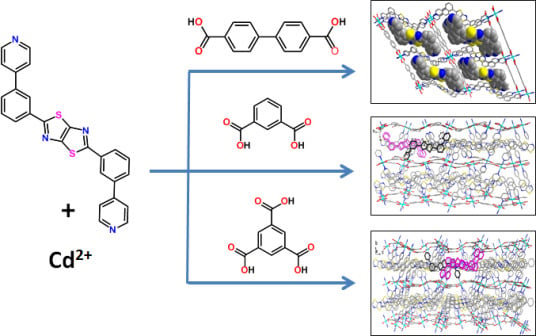
2.1. The Structure of Complex 1
2.2. The Structure of Complex 2
2.3. The Structure of Complex 3
2.4. TGA
2.5. IR Analysis
2.6. UV/Vis Analysis
2.7. Luminescence Properties
3. Experiment
3.1. Material and Physical Measurements
3.2. Synthesis of 3-(Pyridine-4-yl) Benzaldehyde
3.3. Synthesis of 2,5-Bis(3-(pyridine-4-yl)phenyl)thiazolo[5,4-d]thiazole (BPPT)
3.4. Synthesis of [Cd(BPPT)(BPDA)](BPPT)n (1)
3.5. Synthesis of [Cd(BPPT) (IP)] (CH3OH) (2)
3.6. Synthesis of [Cd3(BPPT)3(BTC)2(H2O)2] (3)
3.7. X-Ray Crystallography
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal-organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Wang, X.-S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal-organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; García, H.; Xamena, F.X.L. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Voorde, B.V.; Bueken, B.; Denayer, J.; Vos, D.D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [Green Version]
- Kupgan, G.; Abbott, L.J.; Hart, K.E.; Colina, C.M. Modeling Amorphous Microporous Polymers for CO2 Capture and Separations. Chem. Rev. 2018, 118, 5488–5538. [Google Scholar] [CrossRef]
- Pan, M.; Liao, W.-M.; Yin, S.-Y.; Sun, S.-S.; Su, C.-Y. Single-Phase White-Light-Emitting and Photoluminescent Color-Tuning Coordination Assemblies. Chem. Rev. 2018, 118, 8889–8935.
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Duyne, R.P.V.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Josepha, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.X.; Wang, F.; Zhang, J. Design and synthesis of multifunctional metal–organic zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Coronado, E. Magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar] [CrossRef] [Green Version]
- Vleet, M.J.V.; Weng, T.; Li, X.; Schmidt, J.R. In Situ, Time-Resolved, and Mechanistic Studies of Metal-Organic Framework Nucleation and Growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Woodward, A.N.; Kolesar, J.M.; Hall, S.R.; Saleh, N.A.; Jones, D.S.; Walter, M.G. Thiazolothiazole Fluorophores Exhibiting Strong Fluorescence and Viologen-Like Reversible Electrochromism. J. Am. Chem. Soc. 2017, 139, 8467–8473. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Yang, S.H.; Luo, P.; Li, L.K.; Du, C.X.; Zang, S.Q. Dicarboxylate-Induced Structural Diversity of Luminescent Zn(II)/Cd(II) Metal–Organic Frameworks Based on the 2,5-Bis(4-pyridyl)thiazolo[5,4-d]thiazole Ligand. Eur. J. Inorg. Chem. 2019, 2019, 2725–2734. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, T.T.; Hu, H.M.; Li, C.T.; Zhou, C.S.; Wang, X.; Xue, G. Two luminescent d 10 metal coordination polymers assembled from a semirigid terpyridyl carboxylate ligand with high selective detecting of Cu2+, Cr2O72− and acetone. J. Solid. State. Chem. 2017, 251, 79–89. [Google Scholar] [CrossRef]
- Xu, B.; Lu, J.; Cao, R. Anion-Assisted Structural Variation of Cadmium Coordination Polymers: From 2D → 3D Inclined Polycatenation to 2D → 3D Polythreading. Cryst. Growth Des. 2009, 9, 3003–3005. [Google Scholar] [CrossRef]
- Guo, J.; Sun, D.; Zhang, L.; Yang, Q.; Zhao, X.; Sun, D. 1D Looped Chain, 1D Tube-like Chain, and 3D Inorganic–Organic Hybrid Coordination Polymers Assembled from 1,6-Di(triazole-1-yl-methyl)-4-R-phenol and Cadmium(II) Thiocyanate. Cryst. Growth Des. 2008, 8, 1599–1604. [Google Scholar]
- Biswal, B.P.; Becker, D.; Chandrasekhar, N.; Seenath, J.S.; Paasch, S.; Machill, S.; Hennersdorf, F.; Brunner, E.; Weigand, J.J.; Berger, R.; et al. Exploration of Thiazolo[5,4-d]thiazole Linkages in Conjugated Porous Organic Polymers for Chemoselective Molecular Sieving. Chem. Eur. J. 2018, 24, 10868–10875. [Google Scholar] [CrossRef]
- Lin, H.; Maggard, P.A. Ligand-Mediated Interconversion of Multiply-Interpenetrating Frameworks in CuI/ReVII-Oxide Hybrids. Inorg. Chem. 2009, 48, 8940–8946. [Google Scholar] [CrossRef]
- Li, D.X.; Chen, M.M.; Li, F.L.; Ren, Z.G.; Wu, B.; Lang, J.P. Substituent groups-driven construction of two different Cd(II) coordination polymers from CdSO4, tetrakis(4-pyridyl)cyclobutane and 5-R-1,3-benzenedicarboxylates. Inorg. Chem. Commun. 2013, 35, 302–306. [Google Scholar] [CrossRef]
- Chen, X.L.; Cui, H.L.; Yang, H.; Liu, L.; Wang, X.; Ren, Y.X.; Wang, J.J.; Han, B. A Zinc(II) Coordination Polymer Based on a Polycarboxylate Ligand: Synthesis, Crystal Structure, Photoluminescence and Photocatalysis. Chin. J. Struct. Chem. 2019, 12, 2148–2154. [Google Scholar]
- Wang, X.L.; Qin, C.; Wang, E.B.; Xu, L.; Su, Z.M.; Hu, C.W. Interlocked and interdigitated architectures from self-assembly of long flexible ligands and cadmium salts. Angew. Chem. Int. Ed. 2004, 43, 5036–5040. [Google Scholar] [CrossRef]
- Johnson, J.R.; Rotenberg, D.H.; Ketcham, R. Thiazolothiazoles. II. Parent heterocycle and its carboxylic and amino derivatives. J. Am. Chem. Soc. 1970, 92, 4046–4050. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS 97, Program for the Solution of Crystal Structures; University of Götingen: Götingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL 97, Program for the Refinement of Crystal Structures; University of Götingen: Götingen, Germany, 1997. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |








| Complex | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C66H40CdN8O4S4 | C35H26CdN4O6S2 | C96H61Cd3N12O14S6 |
| Formula weight | 1249.71 | 769.08 | 2113.01 |
| Temperature | 293(2) K | 293(2) K | 293(2) K |
| Wavelength | 1.54178 A | 1.54178 A | 1.54178 A |
| Crystal system, space group | Triclinic, P-1 | Monoclinic, P21/c | Triclinic, P-1 |
| Unit cell dimensions | a = 5.4580(4) Å α = 76.509(5) deg. | a = 12.7996 (2) Å α = 90 deg. | a = 10.4278(4) Å α = 88.931(4) deg. |
| b = 15.3295(9) Å β = 85.570(6) deg. | b = 17.9295(3) Å β = 96.4141(14) deg. | b = 12.5607(7) Å β = 89.037(4) deg. | |
| c = 16.3168(11) Å γ = 82.198(5) deg. | c = 14.5012(2) Å γ = 90 deg. | c = 18.2058(8) Å γ = 83.109(4) deg. | |
| Volume | 1313.77(15) Å3 | 3307.07(8) Å3 | 2366.71(19) Å3 |
| Z, Calculated density | 1, 1.580 Mg/m3 | 4, 1.559 Mg/m3 | 1, 1.483 Mg/m3 |
| Absorption coefficient | 5.324 mm−1 | 6.908 mm−1 | 7.147 mm−1 |
| F(000) | 636 | 1544 | 1057 |
| Crystal size | 0.3 × 0.2 × 0.1 mm | 0.3 × 0.25 × 0.2 mm | 0.3 × 0.2 × 0.14 mm |
| Theta range for data collection | 3.60 to 70.95 deg. | 3.47 to 70.99 deg. | 4.26 to 71.09 deg. |
| Limiting indices | −5 ≤ h ≤ 6, −18 ≤ k ≤ 18, −19 ≤ l ≤ 19 | −15 ≤ h ≤ 11, −21 ≤ k ≤ 21, −16 ≤ l ≤ 17 | −12 ≤ h ≤ 8, −15 ≤ k ≤ 15, −22 ≤ l ≤ 22 |
| Reflections collected/unique | 8494/5078 [R(int) = 0.0331] | 12555/6390 [R(int) = 0.0204] | 16199/9166 [R(int) = 0.0426] |
| Completeness to theta | 97.5% | 98.1% | 97.3% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Refinement method | Full-matrix least-squares on F^2 | Full-matrix least-squares on F^2 | Full-matrix least-squares on F^2 |
| Data/restraints/parameters | 4951/0/376 | 6268/6/433 | 8917/0/592 |
| Goodness-of-fit on F^2 | 0.938 | 1.061 | 1.047 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0407, wR2 = 0.1112 | R1 = 0.0388, wR2 = 0.1085 | R1 = 0.0725, wR2 = 0.2180 |
| R indices (all data) | R1 = 0.0473, wR2 = 0.1167 | R1 = 0.0416, wR2 = 0.1107 | R1 = 0.0797, wR2 = 0.2300 |
| Largest diff. peak and hole | 0.488 and −0.819 | 2.027 and −1.389 e.A−3 | 3.271 and −1.542 e.A−3 |
| Complex 1 | |||||
| Bond | Dist. | Bond | Dist. | Bond | Dist. |
| Cd(1)-O(1) | 2.303(2) | Cd(1)-N(1) | 2.351(3) | Cd(1)-O(2) | 2.461(2) |
| Angle | (°) | Angle | (°) | Angle | (°) |
| O(1)#2-Cd(1)-O(1) | 180.00(12) | O(1)#2-Cd(1)-N(1) | 89.89(9) | O(1)-Cd(1)-N(1) | 90.11(9) |
| O(1)#2-Cd(1)-N(1)#2 | 90.11(9) | O(1)-Cd(1)-N(1)#2 | 89.89(9) | N(1)-Cd(1)-N(1)#2 | 180.0 |
| O(1)#2-Cd(1)-O(2) | 124.89(8) | O(1)-Cd(1)-O(2) | 55.11(8) | N(1)-Cd(1)-O(2) | 89.93(9) |
| N(1)#2-Cd(1)-O(2) | 90.07(9) | O(1)#2-Cd(1)-O(2)#2 | 55.11(8) | O(1)-Cd(1)-O(2)#2 | 124.89(8) |
| N(1)-Cd(1)-O(2)#2 | 90.07(9) | ||||
| Complex 2 | |||||
| Bond | Dist. | Bond | Dist. | Bond | Dist. |
| N(4)-Cd(1)#1 | 2.317(18) | O(3)-Cd(1)#2 | 2.381(17) | Cd(1)-N(4)#3 | 2.317(18) |
| Cd(1)-N(1) | 2.324(18) | Cd(1)-O(2) | 2.364(16) | Cd(1)-O(1)#4 | 2.366(16) |
| Cd(1)-O(3)#5 | 2.381(17) | Cd(1)-O(4)#5 | 2.393(17) | Cd(1)-O(1) | 2.522(16) |
| Angle | (°) | Angle | (°) | Angle | (°) |
| N(4)#3-Cd(1)-N(1) | 176.5(7) | N(4)#3-Cd(1)-O(2) | 94.7(6) | N(1)-Cd(1)-O(2) | 87.4(6) |
| N(4)#3-Cd(1)-O(1)#4 | 90.8(6) | N(1)-Cd(1)-O(1)#4 | 85.7(6) | O(2)-Cd(1)-O(1)#4 | 125.9(5) |
| N(4)#3-Cd(1)-O(3)#5 | 86.5(7) | N(1)-Cd(1)-O(3)#5 | 93.7(7) | O(2)-Cd(1)-O(3)#5 | 140.2(6) |
| O(1)#4-Cd(1)-O(3)#5 | 93.7(6) | N(4)#3-Cd(1)-O(4)#5 | 90.3(7) | N(1)-Cd(1)-O(4)#5 | 92.7(7) |
| O(2)-Cd(1)-O(4)#5 | 85.7(6) | O(1)#4-Cd(1)-O(4)#5 | 148.2(6) | O(3)#5-Cd(1)-O(4)#5 | 54.6(6) |
| N(4)#3-Cd(1)-O(1) | 92.9(6) | N(1)-Cd(1)-O(1) | 86.1(6) | O(2)-Cd(1)-O(1) | 53.3(5) |
| O(1)#4-Cd(1)-O(1) | 72.7(6) | O(3)#5-Cd(1)-O(1) | 166.5(6) | O(4)#5-Cd(1)-O(1) | 139.0(5) |
| Complex 3 | |||||
| Bond | Dist. | Bond | Dist. | Bond | Dist. |
| Cd(1)-O(3) | 2.253(5) | Cd(1)-N(5) | 2.319(6) | Cd(1)-O(8) | 2.346(9) |
| Cd(2)-O(2)#2 | 2.255(4) | Cd(2)-N(2) | 2.303(5) | Cd(2)-O(1) | 2.309(4) |
| Cd(2)-N(1) | 2.325(6) | Cd(2)-O(6)#3 | 2.367(5) | Cd(2)-O(5)#3 | 2.398(5) |
| Angle | (°) | Angle | (°) | Angle | (°) |
| O(3)#1-Cd(1)-O(3) | 180.00(11) | O(3)#1-Cd(1)-N(5)#1 | 88.7(2) | O(3)-Cd(1)-N(5)#1 | 91.3(2) |
| O(3)#1-Cd(1)-N(5) | 91.3(2) | O(3)-Cd(1)-N(5) | 88.7(2) | N(5)#1-Cd(1)-N(5) | 180.0(3) |
| O(3)#1-Cd(1)-O(8)#1 | 82.2(3) | O(3)-Cd(1)-O(8)#1 | 97.8(3) | N(5)#1-Cd(1)-O(8)#1 | 90.0(4) |
| N(5)-Cd(1)-O(8)#1 | 90.0(4) | O(3)#1-Cd(1)-O(8) | 97.8(3) | O(3)-Cd(1)-O(8) | 82.2(3) |
| N(5)#1-Cd(1)-O(8) | 90.0(4) | N(5)-Cd(1)-O(8) | 90.0(4) | O(8)#1-Cd(1)-O(8) | 180.000(1) |
| O(2)#2-Cd(2)-N(2) | 95.93(19) | O(2)#2-Cd(2)-O(1) | 122.65(17) | N(2)-Cd(2)-O(1) | 87.30(18) |
| O(2)#2-Cd(2)-N(1) | 88.3(2) | N(2)-Cd(2)-N(1) | 71.0(2) | O(1)-Cd(2)-N(1) | 83.8(2) |
| O(2)#2-Cd(2)-O(6)#3 | 146.95(17) | N(2)-Cd(2)-O(6)#3 | 90.31(18) | O(1)-Cd(2)-O(6)#3 | 89.97(17) |
| N(1)-Cd(2)-O(6)#3 | 90.4(2) | O(2)#2-Cd(2)-O(5)#3 | 91.94(17) | N(2)-Cd(2)-O(5)#3 | 99.5(2) |
| O(1)-Cd(2)-O(5)#3 | 144.07(16) | N(1)-Cd(2)-O(5)#3 | 88.3(2) | O(6)#3-Cd(2)-O(5)#3 | 55.02(16) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, H.-Y.; Zhang, G.; Yang, M.; Liu, D.-Z.; Yang, Y.-S.; Zhang, Y.-B. Synthesis, Crystal Structures and Luminescence Properties of Three New Cadmium 3D Coordination Polymers. Molecules 2020, 25, 2465. https://doi.org/10.3390/molecules25112465
Ju H-Y, Zhang G, Yang M, Liu D-Z, Yang Y-S, Zhang Y-B. Synthesis, Crystal Structures and Luminescence Properties of Three New Cadmium 3D Coordination Polymers. Molecules. 2020; 25(11):2465. https://doi.org/10.3390/molecules25112465
Chicago/Turabian StyleJu, Hai-Yan, Gang Zhang, Ming Yang, De-Zheng Liu, Yong-Sheng Yang, and Yan-Bo Zhang. 2020. "Synthesis, Crystal Structures and Luminescence Properties of Three New Cadmium 3D Coordination Polymers" Molecules 25, no. 11: 2465. https://doi.org/10.3390/molecules25112465
APA StyleJu, H. -Y., Zhang, G., Yang, M., Liu, D. -Z., Yang, Y. -S., & Zhang, Y. -B. (2020). Synthesis, Crystal Structures and Luminescence Properties of Three New Cadmium 3D Coordination Polymers. Molecules, 25(11), 2465. https://doi.org/10.3390/molecules25112465