Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials
Abstract
:1. Introduction
2. Supercritical Solvent Impregnation (SSI)
2.1. Impregnation of Textiles and Fibers
2.2. Impregnation of Polymeric Forms Other than Textiles and Fibers
3. Supercritical Assisted Impregnation (SAI) and High-Pressure Assisted Impregnation (HPAI)
4. Supercritical Solvent Impregnation or Supercritical Assisted Impregnation Coupled with Polymerization in scCO2
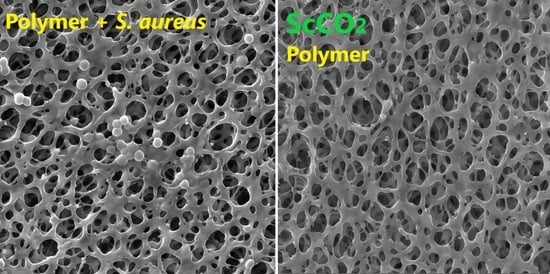
5. Supercritical Foaming
6. Supercritical Drying of Metal-Carrying Gels
7. Other Methodologies Applied to the Development of Antibacterial Materials
8. Discussion
Funding
Conflicts of Interest
References
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 4 March 2020).
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance (AR/AMR). Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 4 March 2020).
- Antibiotic-Resistant Infections Threaten Modern Medicine. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/Threat-Modern-Medicine-508.pdf (accessed on 4 March 2020).
- World Health Organization Regional Office for Europe. Moving Towards a Multisectorial Approach to Tackling Antimicrobial Resistance. Available online: http://www.euro.who.int/en/countries/france/news/news/2020/3/moving-towards-a-multisectoral-approach-to-tackling-antimicrobial-resistance (accessed on 20 March 2020).
- Kiellow, A.W.; Henriksen, O. Supercritical wood impregnation. J. Supercrit. Fluid. 2009, 50, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.; Kjellow, A.W.; Henriksen, O. Modeling and optimization of the supercritical wood impregnation process-Focus on pressure and temperature. J. Supercrit. Fluid. 2012, 66, 307–314. [Google Scholar] [CrossRef]
- Cookson, Q.L. Treatment of eucalypt heartwood using supercritical carbon dioxide as a Carrier. In Proceedings of the 27th Products Research Conference, CSIRO Forestry and Forest Products, Clayton, Australia, 12–13 November 2001. [Google Scholar]
- Iversen, S.B.; Larsen, T.; Henriksen, O.; Felsvang, K. The world’s first commercial supercritical wood treatement plant. In The International Society for the Advancement of Supercritical Fluids, Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28−30 April 2003; Institute National Polytechnique de Lorraine: Vandoeuvre-lès-Nancy, France, 2003. [Google Scholar]
- Van der Kraan, M.; Cid, M.V.F.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.J. Dyeing of natural and synthetic textiles in supercritical carbon dioxide with disperse reactive dyes. J. Supercrit. Fluid. 2007, 40, 470–476. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles—A review. Color. Technol. 2012, 129, 2–17. [Google Scholar] [CrossRef]
- Dyecoo. Available online: http//www.dyecoo.com/ (accessed on 20 March 2020).
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Mišić, D. The applicability of supercritical extracts in clinical treatment of bacterial infections in humans and animals. In Supercritical CO2 Extraction and its Applications; Rój, E., Ed.; Polish Foundations of the Opportunities Industrialization Centers “OIC Poland”: Lublin, Poland, 2014; pp. 23–34. [Google Scholar]
- Cosentino, S.; Tuberoso, C.I.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In vitro antimicrobial activity and chemical composition of Sardinian thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.M.; Cannatelli, A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=thymol (accessed on 25 March 2020).
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluid. 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Radetic, M.; Zizovic, I. Solubility of thymol in supercritical carbon dioxide and its impregnation on cotton gauze. J. Supercrit. Fluid. 2013, 84, 173–181. [Google Scholar] [CrossRef]
- Milovanovic, S.; Radetic, M.; Misic, D.; Asanin, J.; Leontijevic, V.; Ivanovic, J.; Zizovic, I. High pressure modified cotton in wound dressing applications. In Cotton Fibers: Characteristics, Uses and Performance; Gordon, S., Abidi, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 117–205. [Google Scholar]
- Nithyakalyani, D.; Ramachandran, T.; Rajendran, R.; Mahalakshmi, M. Assessment of antibacterial activity of herbal finished surface modified polypropylene non-woven fabric against bacterial pathogens of wound. J. Appl. Polym. Sci. 2013, 129, 672–681. [Google Scholar] [CrossRef]
- Markovic, D.; Milovanovic, S.; Radetic, M.; Jokic, B.; Zizovic, I. Impregnation of corona modified polypropylene non-woven material with thymol in supercritical carbon dioxide for antimicrobial application. J. Supercrit. Fluid. 2015, 101, 215–221. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; De Clerck, K.; Zizovic, I.; Stojanović, D.; Radetić, M. Development of material with strong antimicrobial activity by high pressure CO2 impregnation of polyamide nanofibers with thymol. J. CO2 Util. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluid. 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Misic, D.; Alvarez, M.V.; Jaeger, P.; Zizovic, I.; Eggers, R. Development of polycaprolactone scaffold with antibacterial activity by the integrated supercritical extraction and impregnation process. J. Supercrit. Fluid. 2013, 78, 42–53. [Google Scholar] [CrossRef]
- Ivanovic, J.; Milovanovic, S.; Stamenic, M.; Fanovich, M.A.; Jaeger, P.; Zizovic, I. Application of an Integrated Supercritical Extraction and Impregnation Process for Incorporation of Thyme Extracts into Different Carriers. In Handbook of Supercritical Fluids; Osborne, J., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 257–281. [Google Scholar]
- Zizovic, I.; Jaeger, P.T.; Pietsch, A. Production of materials with antimicrobial properties by a combined supercritical extraction and impregnation process. In Proceedings of the International Symposium on Supercritical Fluids, Antibes-Juan-les-Pins, Valence, France, 22–25 April 2018. [Google Scholar]
- Zizovic, I.; Ivanovic, J.; Milovanovic, S.; Stamenic, M. Impregnations using supercritical carbon dioxide. In Supercritical CO2 Extraction and its Applications; Rój, E., Ed.; Polish Foundations of the Opportunities Industrialization Centers ”OIC Poland”: Lublin, Poland, 2014; pp. 23–34. [Google Scholar]
- Zizovic, I.; Ivanovic, J.; Milovanovic, S.; Adamovic, T. Application of supercritical fluids in development of materials with antibacterial properties. In Supercritical Fluid Applications; Roj, E., Ed.; New Chemical Syntheses Institute: Pulawy, Poland, 2016; pp. 45–60. [Google Scholar]
- Maksimovic, S.; Tadic, V.; Radmanovic, T.; Milovanovic, S.; Stankovic, M.; Zizovic, I. Utilization of the integrated process of supercritical extraction and impregnation for incorporation of Helichrysum italicum extract into corn starch xerogel. Chem. Ind. Chem. Eng. Q. 2018, 24, 191–200. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Zizovic, I.; Misic, D.; Jaeger, P. Functionalization of polycaprolactone/hydroxyapatite scaffolds with Usnea lethariiformis extract by using supercritical CO2. Mater. Sci. Eng. C 2016, 58, 204–212. [Google Scholar] [CrossRef]
- Rój, E.; Tadić, V.M.; Mišić, D.; Zizovic, I.; Arsić, I.; Dobrzyńska-Inger, A.; Kostrzewa, D. Supercritical carbon dioxide hops extracts with antimicrobial properties. Open Chem. 2015, 13, 1157–1171. [Google Scholar] [CrossRef]
- Gittard, S.D.; Hojo, D.; Hyde, G.K.; Scarel, G.; Narayan, R.J.; Parsons, G.N. Antifungal textiles formed using silver deposition in supercritical carbon dioxide. J. Mater. Eng. Perform. 2010, 19, 368–373. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, X.; Zhang, Q. Synthesis of CO2-Philic Polysiloxane with N-Halamine Side Groups for Biocidal Coating on Cotton. Ind. Eng. Chem. Res. 2012, 51, 9260–9265. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Han, Q.; Mi, Y.; Sun, S.; Feng, C.; Xiao, H.; Yu, P.; Yang, C. Synthesis of polysiloxane with 5,5-dimethylhydantoin-based N-halamine pendants for biocidal functionalization of polyethylene by supercritical impregnation. J. Appl. Polym. Sci. 2017, 134, 44721–44729. [Google Scholar]
- Chen, Y.; Yu, P.; Feng, C.; Wang, Y.; Han, Q.; Zhang, Q. Synthesis of polysiloxane with quaternized N-halamine moieties for antibacterial coating of polypropylene via supercritical impregnation technique. Appl. Surf. Sci. 2017, 419, 683–691. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Ren, G.; Feng, C.; Li, N.; Yu, H.; Han, Q. Preparation of biocidal 4-ethyl-4-(hydroxymethyl)oxazolidin-2-one-based N-halamine polysiloxane for impregnation of polypropylene in supercritical CO2. J. Appl. Polym. Sci. 2018, 135, 46624–46631. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Bayraktar, Z.; Kiran, E. Miscibility, Phase Separation, and Volumetric Properties in Solutions of Poly(dimethylsiloxane) in Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2000, 75, 1397–1403. [Google Scholar] [CrossRef]
- O’Neill, M.L.; Cao, Q.; Fang, M.; Johnston, K.P. Solubility of Homopolymers and Copolymers in Carbon Dioxide. Ind. Eng. Chem. Res. 1998, 37, 3067–3079. [Google Scholar] [CrossRef]
- Elmaaty, A.T.; Ma, J.; El-Taweel, F.; El-Aziz, E.A.; Okubayashi, S. Facile Bifunctional Dyeing of Polyester under Supercritical Carbon Dioxide Medium with New Antibacterial Hydrazono Propanenitrile Dyes. Ind. Eng. Chem. Res. 2014, 53, 15566–15570. [Google Scholar] [CrossRef]
- Elmaaty, A.T.; El-Aziz, A.E.; Ma, J.; El-Taawel, F.; Okubayashi, S. Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide. Fibers 2015, 3, 309–322. [Google Scholar] [CrossRef]
- Ma, J.; Elmaaty, A.T.; Okubayashi, S. Effect of Supercritical Carbon Dioxide on Dyeability and Physical Properties of Ultra-High-Molecular-Weight Polyethylene Fiber. AUTEX Res. J. 2019, 19, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohyd. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Adamovic, T.; Aksentijevic, K.; Misic, D.; Ivanovic, J.; Zizovic, I. Cellulose Acetate Based Material with Antibacterial Properties Created by Supercritical Solvent Impregnation. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Zizovic, I.; Senerovic, L.; Moric, I.; Adamovic, T.; Jovanovic, M.; Kalagasidis Krusic, M.; Misic, D.; Stojanovic, D.; Milovanovic, S. Utilization of supercritical carbon dioxide in fabrication of cellulose acetate films with anti-biofilm effects against Pseudomonas aeruginosa and Staphylococcus aureus. J. Supercrit. Fluid. 2018, 140, 11–20. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S.F. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Oppenheimer-Shaanan, Y.; Steinberg, N.; Kolodkin-Gal, I. Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol. 2013, 21, 594–601. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Adamovic, T.; Milovanovic, S.; Markovic, D.; Zizovic, I. Impregnation of cellulose acetate films with carvacrol using supercritical carbon dioxide. Tehnika 2018, 73, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Varona, S.; Rodríguez-Rojo, S.; Martín, A.; José Cocero, M.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluid. 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Senerović, L.; Morić, I.; Zizovic, I. SSI in the antibacterial mats design. Unpublished work. 2020. [Google Scholar]
- Darpentigny, C.; Marcoux, P.R.; Menneteau, M.; Michel, B.; Ricoul, F.; Jean, B.; Bras, J.; Nonglaton, G. Antimicrobial cellulose nanofibril porous materials obtained by supercritical impregnation of thymol. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Terzić, I.; Ivanović, J.; Žižović, I.; Lučić Škorić, M.; Milosavljević, N.; Milašinović, N.; Kalagasidis Krušić, M. A novel chitosan gels: Supercritical CO2 drying and impregnation with thymol. Polym. Eng. Sci. 2018, 58, 2192–2199. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; de Sousa, H.C. Development of natural-based wound dressings impregnated with bioactive compounds and using supercritical carbon dioxide. Int. J. Pharmaceut. 2011, 408, 9–19. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Fukukawa, N.; Sakafuji, J.; Oro, K.; Hata, K.; Nakayama, Y.; Shiono, T. Impregnation of Poly(L-lactide-ran-cyclic carbonate) Copolymers with Useful Compounds with Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2011, 121, 1431–1441. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Yu, J.P.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Preparation of Roxithromycin-Loaded Poly(l-lactic Acid) Films with Supercritical Solution Impregnation. Ind. Eng. Chem. Res. 2011, 50, 13813–13818. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging, Compos. Part B—Eng. 2019, 176. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluid. 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Goni, M.L.; Ganan, N.A.; Strumia, M.C.; Martinia, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercriticalcarbon dioxide impregnation. J. Supercrit. Fluid. 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High pressure carbon dioxide for impregnation of clove essential oil in LLDPE films. Innov. Food Sci. Emerg. 2017, 41, 206–215. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Martínez, F.; Salazar, L.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Assessment of kinetic release of thymol from LDPE nanocomposites by supercritical impregnation: Effect of depressurization rate and nanoclay content. Eur. Polym. J. 2017, 93, 294–306. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Añazco, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Effect of pressure and time on scCO2-assisted incorporation of thymol into LDPE-based nanocomposites for active food packaging. J. CO2 Util. 2018, 26, 434–444. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; da Silva, M.A.; de Sousa, H.C.; Braga, M.E.M.; Kieckbusch, T.G. Influence of natamycin loading methods on the physical characteristics of alginate active films. J. Supercrit. Fluid. 2013, 76, 74–82. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocompositefilms using supercritical fluid technology for the development of foodactive packaging. Carbohyd. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Milovanovic, S.; Jankovic-Castvan, I.; Ivanovic, J.; Zizovic, I. Effect of Starch Xero- and Aerogels Preparation on the Supercritical CO2 Impregnation of Thymol. Starch—Starke 2015, 67, 174–182. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Ren, G.; Zhang, Q.; Han, Q.; Teng, H. Interpenetration of Polyethylene Terephthalate with Biocidal Quaternary Ammonium /N-Chloramine Polysiloxane in Supercritical CO2. Ind. Eng. Chem. Res. 2017, 56, 9560–9568. [Google Scholar] [CrossRef]
- Xu, W.Z.; Yang, L.; Charpentier, P.A. Preparation of Antibacterial Softwood via Chemical Attachment of Quaternary Ammonium Compounds Using Supercritical CO2. ACS Sustain. Chem. Eng. 2016, 4, 1551–1561. [Google Scholar] [CrossRef]
- Renner, M.; Weidner, E.; Brandin, G. High-pressure carbon dioxide tanning. Chem. Eng. Res. Des. 2009, 87, 987–996. [Google Scholar] [CrossRef]
- Mölders, N.; Renner, M.; Errenst, C.; Weidner, E. Incorporation of antibacterial active additives inside polycarbonatesurfaces by using compressed carbon dioxide as transport aid. J. Supercrit. Fluid. 2018, 132, 83–90. [Google Scholar] [CrossRef]
- Niu, A.; Han, Y.; Wu, J.; Yu, N.; Xu, Q. Synthesis of One-Dimensional Carbon Nanomaterials Wrapped by Silver Nanoparticles and Their Antibacterial Behavior. J. Phys. Chem. C 2010, 114, 12728–12735. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kim, B.K.; Jo, Y.L.; Shim, J.J. Ag@graphene oxide nanocomposite as an efficient visible-light plasmonic photocatalyst for the degradation of organic pollutants: A facile green synthetic approach. Mater. Chem. Phys. 2014, 143, 1452–1461. [Google Scholar] [CrossRef]
- Karthäuser, J. Method of Producing an Interpenetrating Polymer Network (IPN), the IPN and Use Thereof. U.S. Patent No. US7687585 B2, 30 March 2010. [Google Scholar]
- Steffensen, S.L.; Vestergaard, M.H.; Groenning, M.; Alm, M.; Franzyk, H.; Nielsen, H.M. Sustained prevention of biofilm formation on a novel silicone matrix suitable for medical devices. Eur. J. Pharm. Biopharm. 2015, 94, 305–311. [Google Scholar] [CrossRef]
- Steffensen, S.L.; Vestergaard, M.H.; Moller, E.H.; Groenning, M.; Alm, M.; Franzyk, H.; Nielsen, H.M. Soft hydrogels interpenetrating silicone-a polymer network for drug-releasing medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 104, 402–410. [Google Scholar] [CrossRef]
- Stenger, M.; Klein, K.; Grønnemose, R.B.; Klitgaard, J.K.; Kolmos, H.J.; Lindholt, J.S.; Alm, M.; Thomsen, P.; Andersen, T.E. Co-release of dicloxacillin and thioridazine from catheter material containing an interpenetrating polymer network for inhibiting device-associated Staphylococcus aureus infection. J. Control. Release 2016, 241, 125–134. [Google Scholar] [CrossRef]
- Maki, D.G.; Tambyah, P.A. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 2001, 7, 342–347. [Google Scholar] [CrossRef]
- Schumm, K.; Lam, T.B.L. Types of urethral catheters for management of shortterm voiding problems in hospitalized adults: A short version cochrane review. Neurourol. Urodyn. 2008, 27, 110–121. [Google Scholar] [CrossRef]
- Raad, I.; Hanna, H.; Maki, D. Intravascular catheter-related infections: Advances in diagnosis, prevention, and management. Lancet Infect. Dis. 2007, 7, 645–657. [Google Scholar] [CrossRef]
- Dimick, J.B.; Pelz, R.K.; Consunji, R.; Swoboda, S.M.; Hendrix, C.W.; Lipsett, P.A. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch. Surg. 2001, 136, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, V.G.; Bonifacio, V.D.B.; Raje, V.P.; Casimiro, T.; Moutinho, G.; da Silva, C.L.; Pinho, M.G.; Aguiar-Ricardo, A. Oxazoline-Based Antimicrobial Oligomers: Synthesis by CROP Using Supercritical CO2. Macromol. Biosci. 2011, 11, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Correia, V.G.; Ferraria, A.M.; Pinho, M.G.; Aguiar-Ricardo, A. Antimicrobial Contact-Active Oligo(2-oxazoline)s-Grafted Surfaces for Fast Water Disinfection at the Point-of-Use. Biomacromolecules 2015, 16, 3904–3915. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides, Innate Immunity, and the Normally Sterile Urinary Tract. J. Am. Soc. Nephrol. 2007, 18, 2810–2816. [Google Scholar] [CrossRef] [Green Version]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 sorption kinetics and thymol impregnation of PCL and PCL-HA. J. Supercrit. Fluid. 2016, 107, 486–498. [Google Scholar] [CrossRef]
- García-González, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial Properties and Osteogenicity of Vancomycin-Loaded Synthetic Scaffolds Obtained by Supercritical Foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovica, J. Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous Cellulose as Metal Nanoparticles Support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Muller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Raman, S.P.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H.; et al. Alginate aerogels carrying calcium, zinc and silver cations for woundcare: Fabrication and metal detection. J. Supercrit. Fluid. 2019, 153, 104545. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tewari, P.H.; Hunt, A.J.; Lofftus, K.D. Ambient-temperature supercritical drying of transparent silica aerogels. Mater. Lett. 1985, 3, 363–367. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of biopolymer aerogels using green solvents. J. Vis. Exp. 2016, 113, e54116. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhong, J.; Zhu, H.; Yang, Y.; Ding, M.; Luo, L.; Huo, Y.; Li, H. Hybrid Cu2O/TiO2 Nanocomposites with Enhanced Photocatalytic Antibacterial Activity toward Acinetobacter baumannii. ACS Appl. Bio Mater. 2019, 2, 4892–4903. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachon, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. Fem. Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef] [Green Version]
- Sui, R.H.; Charpentier, P. Synthesis of metal oxide nanostructures by direct sol-gel chemistry in supercritical fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef]
- Sahraneshin, A.; Takami, S.; Hojo, D.; Minami, K.; Arita, T.; Adschiri, T. Synthesis of shape-controlled and organic-hybridized hafnium oxide nanoparticles under sub- and supercritical hydrothermal conditions. J. Supercrit. Fluid. 2012, 62, 190–196. [Google Scholar] [CrossRef]
- Bhartia, B.; Puniredd, S.R.; Jayaraman, S.; Gandhimathi, C.; Sharma, M.; Kuo, Y.C.; Chen, C.H.; Reddy, V.J.; Troadec, C.; Srinivasan, M.P. Highly Stable Bonding of Thiol Monolayers to Hydrogen-Terminated Si via Supercritical Carbon Dioxide: Toward a Super Hydrophobic and Bioresistant Surface. ACS Appl. Mater. Interfaces 2016, 8, 24933–24945. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Zhao, L.; Yonezawa, S.; Iwai, Y. Modification of the surface of cotton with supercritical carbon dioxide and water to support nanoparticles. J. Supercrit. Fluid. 2012, 61, 199–205. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Martínez-Casado, F.J.; Cheda, J.A.R.; Redondo, M.I.; Pando, C.; Cabañas, A. Production and Characterization of a New Copper(II) Propanoate-Isonicotinamide Adduct Obtained via Slow Evaporation and using Supercritical CO2 as an Antisolvent. Cryst. Growth Des. 2019, 19, 620–629. [Google Scholar] [CrossRef]
- Imbuluzqueta, E.; Elizondo, E.; Gamazo, C.; Moreno-Calvo, E.; Veciana, J.; Ventosa, N.; Blanco-Prieto, M.J. Novel bioactive hydrophobic gentamicin carriers for the treatment of intracellular bacterial infections. Acta Biomater. 2011, 7, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Saelo, S.; Assatarakul, K.; Sane, A.; Suppakul, P. Fabrication of Novel Bioactive Cellulose-Based Films Derived from Caffeic Acid Phenethyl Ester-Loaded Nanoparticles via a Rapid Expansion Process: RESOLV. J. Agric. Food Chem. 2016, 64, 6694–6707. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Rodríguez Rojo, S.; Martín, Á.; Cocero, M.J.; Serra, A.T.; Crespo, T.; Duarte, C.M.M. Antimicrobial activity of lavandin essential oil formulations against three pathogenic food-borne bacteria. Ind. Crop. Prod. 2013, 42, 243–250. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluid. 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Santo, I.E.; Campardelli, R.; Albuquerque, E.C.; de Melo, S.V.; Della Porta, G.; Reverchon, E. Liposomes preparation using a supercritical fluid assisted continuous process. Chem. Eng. J. 2014, 249, 153–159. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J. CO2 Util. 2019, 31, 119–127. [Google Scholar] [CrossRef]
- Trucillo, P.; Ferrari, P.F.; Campardelli, R.; Reverchon, E.; Perego, P. A Supercritical Assisted Process for the Production of Amoxicillin Loaded Liposomes for Anti-microbial Applications. J. Supercrit. Fluid. 2020. [Google Scholar] [CrossRef]
- Campardelli, R.; Trucillo, P.; Reverchon, E. Supercritical assisted process for the efficient production of liposomes containing antibiotics for ocular delivery. J. CO2 Util. 2018, 25, 235–241. [Google Scholar] [CrossRef]
- Trucillo, P.; Cardea, S.; Baldino, L.; Reverchon, E. Production of liposomes loaded alginate aerogels using two supercritical CO2 assisted techniques. J. CO2 Util. 2020, 39, 101161. [Google Scholar] [CrossRef]
- Weidner, E. Impregnation via Supercritical Fluids—Principles and Applications. In Proceedings of the 10th International Symposium on Supercritical Fluids (ISSF 2012), San Francisco, CA, USA, 13–16 May 2012. [Google Scholar]




| Active Substance | Technique and Main Process Parameters | Solid Material | Loading (Result) | Microorganism | Reference |
|---|---|---|---|---|---|
| Thymol | SSI, 35 °C, 15.5 MPa, 1–24 h | Cotton fibers | 1.74–19.6% | E. coli, S. aureus, B. subtilis, E. faecalis, C. albicans | [22] |
| Carvacrol | SSI, 50 °C, 10–30 MPa, 1–24 h | Cotton fibers | 4–14.4% | E. coli, S. aureus | [23] |
| Thymol | SSI, 35 °C, 15.5 MPa, 4 h | Polypropylene fibers | 0.5–11.2% | E. coli, S. aureus, C. albicans | [25] |
| Thymol | SSI, 35 °C, 10 and 20 MPa, 0.5–4 h Near-critical, 25 °C, 7 MPa, 0.5–4 h | Polyamide nanofibers | 22.6–59.2% 6.51–33.8% | E. coli, S. aureus, C. albicans | [26] |
| Mango leaf extract | SSI, 35 and 55 °C, 40 and 50 MPa, 22 h Methanol cosolvent | Polyester fibers | 1.1–2.8% polyphenols | E. coli | [27] |
| Thyme extract | SFE-SSI, 35 °C, 15 MPa, batch 5 h | Cotton fibers Cellulose acetate Polypropylene fibers PCL Chitosan | 7.18% 1.44% 4.78% 9.04% 0.96% | [29] | |
| Usnea barbata extract Curry plant Lemon balm | SFE-SSI, 40 °C, 30 MPa, batch 5 h | LDPE Polypropilene fibers Cotton fibers | 3.05% 3.99% 2.24% | [30] | |
| Hop extract | SFE-SSI, 35 °C, 15 MPa, batch 5 h SFE-SSI, 50 °C, 29 MPa, batch 5 h | PCL Polypropylene fibers Starch xerogel | 6.04% 4.36% 2.58% | [31] | |
| Thyme extract Thymol Thymol Thymol | SFE-SSI, 110 °C, 30 MPa, 2 h batch + 2 h flow SSI, 35–110 °C, 10 and 30 MPa, 2–4 h SSI, 35 °C, 7.5 MPa, 2 h SSI, 35 °C, 15 and 30 MPa, 2 h | PLA PLA PLGA Starch | 1.2% 4.9–6.6% 3.0% 14.7–31.9% | [32] | |
| Ag(hepta), Ag(cod)(hfac) | SSI, 40 °C, 21 MPa, 10–15 h Reduction in H2 + scCO2 | Cotton fabric | Silver coating | C. albicans | [36] |
| N-halamine polysiloxane | SSI, 50 °C, 25 MPa, 3 h | Cotton fibers | 60 nm coating | E. coli, S. aureus | [37] |
| N-halamine polysiloxane | SSI, 50 °C, 28 MPa, overnight | Polyethylene fibers | 73 nm coating | E. coli, S. aureus | [38] |
| N-halamine polysiloxane | SSI, 50 °C, 28 MPa, overnight | Polypropylene fibers | Coating | E. coli, S. aureus | [39,40] |
| Hydrazono propanenitrile dyes | SSI, 120 °C, 15 MPa, 1–3 h Methanol cosolvent | Polyester fabric | Dyeing | E. coli, S. aureus | [44] |
| Hydrazono propanenitrile dyes | SSI, 80–120 °C, 5–15 MPa, 1–3 h | Polyamide fabric | Dyeing | E. coli, S. aureus, P. aeruginosa, B. subtilis | [45] |
| Hydrazono propanenitrile dyes | SSI, 120 °C, 20 MPa, 1–3 h With or without decalin cosolvent | UHMW polyethylene fiber | Dyeing | E. coli, S. aureus, B. cereus | [46] |
| Thymol | SSI, 35 °C, 10 and 20 MPa, 2–45 h | Cellulose acetate | 5–72% | S. aureus, C. albicans | [20] |
| Thymol | SSI, 35 °C, 10 MPa, 2–32 h | Cellulose acetate | 5–66% | S. Typhimurium, S. Enteritidis, L. monocytogenes, L. ivanovii, L. innocua, Corynebacterium, R. equi, B. anthracis, B. cereus, B. subtilis, S. pneumoniae, S. pyogenes, S. aureus, MRSA, K. pneumoniae, P. aeruginosa, E. coli, Acinetobacter, P. mirabilis | [47] |
| Carvacrol | SSI, 50 °C, 10–30 MPa, 2–18 h | Celulose acetate | 5–60% | MRSA, E.coli, Acinetobacter, B. anthracis, B. cereus, B. subtilis, Corynebacterium, K. pneumoniae, L. ivanovii, L. monocytogenes, R. equi, S. Enteritidis, S. pyogenes, S. pneumoniae | [48] |
| Thymol | SSI, 35 °C, 15.5 MPa, 0.5–16 h | Cellulose acetate | 8–64% | S. aureus, MRSA, P. aeruginosa | [49] |
| Carvacrol | SSI, 50 °C, 21 MPa, 0.5 and 2 h | Cellulose acetate | 2.5–31.4% | [54] | |
| Thymol | SSI, 40 °C, 10 MPa, 1 h | Cellulose nanofibril mats | 4.1–8.3% | E. coli, S. epidermidis, C. albicans | [57] |
| Thymol | SSI, 35 °C, 10 MPa, 2–6 h | Chitosan-itaconic acid-methacrylic acid | 1.0–4.6% | [58] | |
| Thymol | SSI, 40 °C, 20 MPa, 3 h Near-critical, 30 °C, 10 MPa, 3 h | N-carboxybutylchitosan Agarose | 0.8–2.5% | [59] | |
| d-limonene | SSI, 40 °C, 20 MPa, 3 h | Poly(L-lactide-ran-cyclic carbonate) | 0.15–5.3% | [60] | |
| Roxithromycin | SSI, 40–70 °C, 8–30 MPa, 0.5–4 h | PLA | 0.5–10.5% | [62] | |
| Thymol | SSI, 40 °C, 9 and 12 MPa, 3 h | PLA | 13.5–20.5% | [63] | |
| Cinnamaldehyde | SSI, 40 °C, 9 and 12 MPa, 3 h | PLA | 8–13% | E. coli, S. aureus | [64] |
| Thymol Cinnamaldehyde | SSI, 40 °C, 12 MPa, 3 h | PLA+nanoclay | 17% 11% | E. coli, S. aureus | [65] |
| Thymol Thyme extract | SSI, 40 °C, 10 MPa, 1–15 h SFE-SSI, 40 °C, 10 MPa, 2–6 h | PLA/PCL | 8–35.8% 4.3–5% | E. coli, B. subtilis | [66] |
| Usnea lethariiformis extract | SFE-SSI, 40 °C/30 MPa SFE; 35 °C/15 MPa SSI; 2 h flow + 1 h circ. | PCL | 0.2–2.8% | MRSA, L. innocua | [28] |
| Usnea lethariiformis extract | SFE-SSI, 40 °C/30 MPa SFE; 35 °C/17 MPa SSI; 2 h flow + 1 h circ. | PCL+hydrohyapatite | 1.7–5.9% | MRSA | [34] |
| Thymol | SSI and near-critical, 40 °C, 7–12 MPa, 4 h | LLDPE | 1.5–3.8% | [67] | |
| Eugenol | SSI, 40 °C, 10–15 MPa, 4 h | LLDPE | 1–6% | [68] | |
| Clove bud essential oil | SSI, 25–45 °C, 15 and 25 MPa, 4 h | LLDPE | 1–4% | [69] | |
| Thymol | SSI, 40 °C, 12 MPa, 1 h | LDPE+nanoclay | 0.36–1.19% | [70] | |
| Thymol | SSI, 40 °C, 9–12 MPa, 0.5–5 h | LDPE+nanoclay | 0.82–1.62% | S. aureus, E. coli | [71] |
| Natamycin | SSI, 40 °C, 20 MPa, 2.5–14 h with or without ethanol cosolvent | Alginate | 0.3–1.6% | [72] | |
| Cinnamaldehyde | SSI, 35 °C, 15 and 2 MPa, 3 h | Starch | 0.1–0.25% | [73] | |
| Thymol | SSI, 35 °C, 15.5, 24 h | Starch | 1.15–4.02% | [74] | |
| Curry plant extract | SFE-SSI, 40 °C, 35 MPa, 5 h | Starch | 1.26% | [33] | |
| Lavandin essential oil | SSI, 40–50 °C, 10–12 MPa, 2 h | n-octenyl succinate modified starch | 2.5–15% | [55] | |
| Quaternary ammonium/N-chloramine polysiloxane | SSI, 50 °C, 28 MPa, overnight | PET | 70 nm coating | S. aureus, E. coli | [75] |
| Quaternary ammonium compounds | SSI and chemical reaction, 100 °C, 41.4 MPa, 20 h Hexamethylene diisocyanate as a linker | Softwood | E. coli | [76] | |
| Silver nitrate | HPAI, 20 °C, 12 MPa, 10 min SAI, 40 and 80 °C, 12 MPa, 10 min Ethanol solution of AgNO3 | Polycarbonate | 2.4 mg/kg 23.4 mg/kg | E. coli | [78] |
| Silver NPs (AgNO3 precursor) | SAI, 65 °C, 12 MPa, 3 h Ethanol solution, glucose as a reducer | Carbon nanomaterials | E.coli | [79] | |
| Silver NPs (AgNO3 precursor) | SAI, 65 °C, 12 MPa, 3 h Ethanol solution, glucose as a reducer | Graphene oxide | E. coli, S. aureus, L. anguillarum | [80] | |
| Ciprofloxacin loaded in IPN material | SSI or SAI + Polymerization SSI/SAI, 40 °C, 20–25 MPa, 20 min–16 h Polymerization, 75 °C, 30–36 MPa, 3 h | IPN material based on silicone elastomer and PHEMA | 13–38% PHEMA | S. aureus | [82] |
| Ciprofloxacin loaded in IPN material | SSI + polymerization SSI, 40 °C, 20 MPa, 16 h | IPN material based on PDMS and PHEMA | 25% PHEMA | S. aureus | [83] |
| Polymerization, 75 °C, 30 MPa, 3 h | |||||
| Dicloxacillin Dicloxacillin and thioridazine | SSI + polymerization SSI, 40 °C, 20–25 MPa, 16 h Polymerization, 75 °C, 30 MPa, 3 h | IPN material based on silicone elastomer and PHEMA | 25.29–41.68% PHEMA | S. aureus, MRSA | [84] |
| 2-oxazoline-based oligomers | SSI + polymerization SSI, 40 °C, 18 MPa, 24 h Polymerization, 65 °C, 18 MPa, 20 h Reaction with tertiary amine, 40 °C, 18 MPa, 20 h | Chitosan | E.coli, S. aureus | [91] | |
| Vancomycin | Foaming from solid dispersion | PCL and chitosan | 1–5% | E. coli, S. aureus | [95] |
| 40 °C, 14 MPa, 1 h | |||||
| Thymol | SSI+ foaming in one step, 35 and 40 °C, 10–30 MPa, 2 h | PCL PCL+hydroxyapatite | 12–18% | [94] | |
| Thymol | SSI + foaming in one step, 25–50 °C, 7.5–15 MPa, 2–24 h | PLA PLGA | 0.92–6.62% | [96] | |
| Silver, gold and platinum NPs | Sc drying of metal-carrying gels Two steps: 5.3 MPa, 4 °C for 6 h, and 10 MPa, 40 °C for 0.5 h | Cellulose | Aerogel containing metal particles | [97] | |
| Ca-Zn Ca-Zn-Ag | High-pressure gelation, 50 MPa, 24 h, room temperature; Sc drying of metal-carrying gel at 50 °C, 12 MPa, 2 h, 20 g/min CO2 flowrate | Calcium-alginate | Aerogel containing metal particles | [99] | |
| TiO2 NPs | Sc drying of metal-carrying gel at 50–60 °C, 11–13 MPa, 5 h, 0.2 kg/h CO2 flowrate | Pectin | Aerogel containing NPs | E. coli | [100] |
| Cu2O and TiO2 | Sc solvothermal process in ethanol as supercrit. fl., 243 °C, 6.4 MPa, 70 min | Cu2O-TiO2 nanocomposites | A. baumannii, P. aeruginosa, E. coli, S. aureus | [104] | |
| Alkylthiols | Sc CO2 grafting at 100 °C and 10 MPa, 120 min | Oxide-free silicon | Deposited monolayer | [108] | |
| TiO2 NPs | Physical treatment of fibers, 40 °C, 20 MPa, 60 min, fast decompression 0.80 MPa/min−1 | Cotton fibers | NPs modified cotton | [109] | |
| Isonicotinamide and copper(II) propanoate | Antisolvent precipitation (SAS)–ethanol soluton, 40 °C, 10 MPa, 1 mL/min | Ligand crystals produced | [110] | ||
| Gentamicin | Antisolvent precipitation from acetone solution at 10 MPa, 25 °C | GEN-AOT complex | Micronized solid | E. coli | [111] |
| Caffeic acid phenethyl ester | RESOLV–ethanol solution at 17.3 MPa and 50 °C; nozzle at 80 °C | NPs produced | P. aeruginosa, C. albicans, L. monocytogenes | [112] | |
| Lavandin essential oil | PGSS drying, 104–130 °C, 6–10 MPa PGSS, 70 °C, 6–8.5 MPa | Soybean lecithin, n-octenyl succinic anhydride modified starch, PCL | Oil encapsulated in polymer | E. coli, S. aureus, B. Baccereus | [113] |
| Lavandin essential oil | PGSS, 76–84 °C, 5.4–8.5 MPa PGSS drying, 108–127 °C, 9–12.4 MPa | PEG n-octenyl succinic anhydride modified starch | Oil encapsulated in polymer | [114] | |
| Vancomycin | SuperLip, 40 °C, 10 MPa | Phospholipids | Liposomes | [116] | |
| Amoxicillin | SuperLip, 40 °C, 10 MPa | Phospholipids | Liposomes | E. coli | [117] |
| Ampicillin Ofloxacin | SuperLip, 40 °C, 10 MPa | Phospholipids | Liposomes | [118] | |
| Amoxicillin | SuperLip, 40 °C, 10 MPa Sc Drying, 35 °C, 20 MPa, 6 h, 1 kg/h scCO2 flowrate | Phospholipids Alginate | Liposomes entrapped in aerogel | [119] |
| High-Pressure Methodology | Reference |
|---|---|
| Supercritical Solvent Impregnation (SSI) | [20,22,23,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,44,45,46,47,48,49,54,55,57,58,59,62,63,64,65,66,67,68,69,70,71,72,73,74,75,94,96] |
| Supercritical Assisted Impregnation (SAI) High-pressure Assisted Impregnation (HPAI) | [78,79,80] [78] |
| SSI/SAI + polymerization | [82,83,84,91] |
| SSI/SAI + chemical reaction other than polymerization | [76,91,108] |
| Supercritical foaming | [28,34,94,95,96] |
| Supercritical drying | [97,99,100,119] |
| Supercritical solvothermal process 1 | [104] |
| Antisolvent techniques | [110,111] |
| RESOLV | [112] |
| PGSS | [113,114] |
| Physical surface modification | [109] |
| Liposome formation (SuperLip) | [116,117,118,119] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizovic, I. Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules 2020, 25, 2491. https://doi.org/10.3390/molecules25112491
Zizovic I. Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules. 2020; 25(11):2491. https://doi.org/10.3390/molecules25112491
Chicago/Turabian StyleZizovic, Irena. 2020. "Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials" Molecules 25, no. 11: 2491. https://doi.org/10.3390/molecules25112491
APA StyleZizovic, I. (2020). Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules, 25(11), 2491. https://doi.org/10.3390/molecules25112491