Mechanochemical Preparation of Pd(II) and Pt(II) Composites with Carbonaceous Materials and Their Application in the Suzuki-Miyaura Reaction at Several Energy Inputs
Abstract
:1. Introduction
2. Results and Discussion
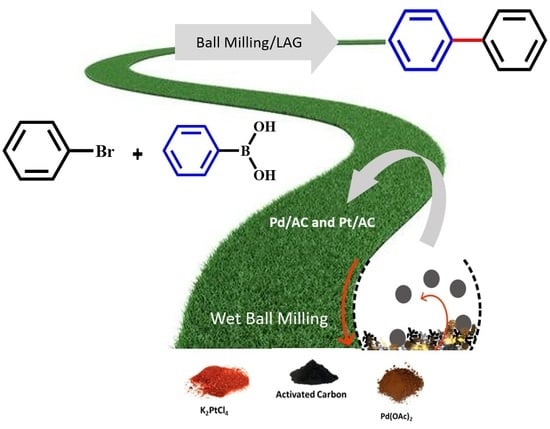
2.1. Preparation of Pd and Pt Catalysts
2.2. Characterization of the Pd/AC and Pt/AC Composites
2.3. Catalytic Studies
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
3.3. Catalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds (Nobel lecture). Angew. Chem. Int. Ed. 2011, 50, 6723–6737. [Google Scholar] [CrossRef] [PubMed]
- Lasri, J.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Charmier, M.A.J.; Pombeiro, A.J.L. Metal-Free and PdII-promoted [2 + 3] cycloadditions of a cyclic nitrone to phthalonitriles: Syntheses of oxadiazolines as well as phthalamide–PdII and dihydropyrrolyl-iminoisoindolinone–PdII complexes with high catalytic activity in Suzuki–Miyaura cross-coupling reactions. Chem. Eur. J. 2008, 14, 9312–9322. [Google Scholar] [CrossRef]
- Lasri, J.; Guedes da Silva, M.F.C.; Kopylovich, M.N.; Mukhopadhyay, S.; Charmier, M.A.J.; Pombeiro, A.J.L. PdII-promoted [2 + 3] cycloaddition of pyrrolin N-oxide to organonitriles. Application of fused bicyclic (Δ4-1,2,4-oxadiazoline)-PdII complexes in the microwave-assisted Suzuki-Miyaura cross-coupling in aqueous medium. Dalton Trans. 2009, 12, 2210–2216. [Google Scholar] [CrossRef]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Kohler, K.; Heidenreich, R.G.; Soomro, S.S.; Prockl, S.S. Supported palladium catalysts for Suzuki reactions: Structure-property relationships, optimized reaction protocol and control of palladium leaching. Adv. Synth. Catal. 2008, 350, 2930–2936. [Google Scholar] [CrossRef]
- Diallo, A.K.; Ornelas, C.; Salmon, L.; Aranzaes, J.R.; Astruc, D. “Homeopathic” catalytic activity and atom-leaching mechanism in Miyaura–Suzuki reactions under ambient conditions with precise dendrimer-stabilized Pd nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 8644–8648. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casco, M.E.; Kirchhoff, S.; Leistenschneider, D.; Rauche, M.; Brunner, E.; Borchardt, L. Mechanochemical synthesis of N-doped porous carbon at room temperature. Nanoscale 2019, 11, 4712–4718. [Google Scholar] [CrossRef] [Green Version]
- Alegria, E.C.B.A.; Fontolan, E.; Ribeiro, A.P.C.; Kopylovich, M.N.; Domingos, C.; Ferraria, A.M.; Bertani, R.; Botelho do Rego, A.M.; Pombeiro, A.J.L. Simple solvent-free preparation of dispersed composites and their application as catalysts in oxidation and hydrocarboxylation of cyclohexane. Mat. Today Chem. 2017, 5, 52–62. [Google Scholar] [CrossRef]
- Fontolan, E.; Alegria, E.C.B.A.; Ribeiro, A.P.C.; Kopylovich, M.N.; Bertani, R.; Pombeiro, A.J.L. Ball milling as an effective method to prepare magnetically recoverable heterometallic catalysts for alcohol oxidation. Inorg. Chim. Acta 2017, 455, 653–658. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Alegria, E.C.B.A.; Ferraria, A.M.; Botelho do Rego, A.M.; Kopylovich, M.N.; Pombeiro, A.J.L. Comparison of microwave and mechanochemical energy inputs in the catalytic oxidation of cyclohexane. Dalton Trans. 2018, 47, 8193–8198. [Google Scholar] [CrossRef] [PubMed]
- Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. Non-Covalent Interactions in the Synthesis and Design of New Compounds; John Wiley & Sons: Chichester, UK, 2016; p. 480. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Noncovalent Interactions in Catalysis; RCS Publishing: Marshfield, MO, USA, 2019; p. 653. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Fontolan, E.; Alegria, E.C.B.A.; Kopylovich, M.N.; Bertani, R.; Pombeiro, A.J.L. The influence of multiwalled carbon nanotubes and graphene oxide additives on the catalytic activity of 3d metal catalysts towards 1-phenylethanol oxidation. J. Mol. Cat. A. Chem. 2017, 426, 557–563. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-free carbocatalysis in advanced oxidation reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons: Chichester, UK, 2009; p. 579. [Google Scholar] [CrossRef]
- Serp, P.; Machado, B. Nanostructured Carbon Materials for Catalysis; RCS Publishing: Marshfield, MO, USA, 2015; p. 555. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R.; et al. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Guerra, J.; Herrero, M.A. Hybrid materials based on Pd nanoparticles on carbon nanostructures for environmentally benign C–C coupling chemistry. Nanoscale 2010, 2, 1390–1400. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojedad, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Leistenschneider, D.; Zürbes, K.; Schneidermann, C.; Grätz, S.; Oswald, S.; Wegner, K.; Klemmed, B.; Giebeler, L.; Eychmüller, A.; Borchardt, L. Mechanochemical functionalization of carbon black at room temperature. J. Carbon Res. 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Rak, M.J.; Friščić, T.; Moores, A. Mechanochemical synthesis of Au, Pd, Ru and Re nanoparticles with lignin as a bio-based reducing agent and stabilizing matrix. Faraday. Discuss. 2014, 170, 155–167. [Google Scholar] [CrossRef]
- Jüntgen, H. Activated carbon as catalyst support: A review of new research results. Fuel. 1986, 65, 1436–1446. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Irmak, S.; Hesenov, A.; Erbatur, O. Preparation of activated carbon supported Pt catalysts and optimization of their catalytic activities for hydrogen gas production from the hydrothermal treatment of biomass-derived compounds. Int. J. Hydrogen Energ. 2012, 37, 17844–17852. [Google Scholar] [CrossRef]
- Arevalo-Bastante, A.; Martin-Martinez, M.; Álvarez-Montero, M.A.; Rodriguez, J.J.; Gómez-Sainero, L.M. Properties of carbon-supported precious metals catalysts under reductive treatment and their influence in the hydrodechlorination of dichloromethane. Catalysts 2018, 8, 664. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liang, Y.; Lu, Z.; Lou, H.; Zhang, X.; Liu, S.; Zheng, B.; Liu, R.; Fu, R.; Wu, D. Mechanochemistry: A green, activation-free and top-down strategy to high-surface-area carbon materials. ACS Sustain. Chem. Eng. 2017, 5, 8535–8540. [Google Scholar] [CrossRef]
- Monguchi, Y.; Fujita, Y.; Hashimoto, S.; Ina, M.; Takahashi, T.; Ito, R.; Nozaki, K.; Maegawa, T.; Sajiki, H. Palladium on carbon-catalyzed solvent-free and solid-phase hydrogenation and Suzuki-Miyaura reaction. Tetrahedron 2011, 67, 8628–8634. [Google Scholar] [CrossRef]
- Hernandez, J.G.; Friscic, T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015, 56, 4253–4265. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Li, Z.-H.; Yu, J.-B.; Su, W.-K. Liquid-assisted grinding accelerating: Suzuki−Miyaura reaction of aryl chlorides under high-speed ball-milling conditions. J. Org. Chem. 2016, 81, 10049–10055. [Google Scholar] [CrossRef]
- Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Solid-state Suzuki–Miyaura cross-coupling reactions: Olefin-accelerated C–C coupling using mechanochemistry. Chem. Sci. 2019, 10, 8202–8210. [Google Scholar] [CrossRef] [Green Version]
- Gratz, S.; Wolfrum, B.; Borchardt, L. Mechanochemical Suzuki polycondensation—from linear to hyperbranched polyphenylenes. Green Chem. 2017, 19, 2973–2979. [Google Scholar] [CrossRef]
- Vogt, C.G.; Gratz, S.; Lukin, S.; Halasz, I.; Etter, M.; Evans, J.D.; Borchardt, L. Direct mechanocatalysis: Palladium as milling media and catalyst in the mechanochemical Suzuki polymerization. Angew. Chem. Int. Ed. 2019, 58, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryanarayana, C. Mechanical alloying and milling. Progress. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mat. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Sopicka-Lizer, M. High-Energy Ball Milling: Mechanochemical Processing of Nanopowders; Woodhead Publishing Limited: Cambridge, UK, 2010; p. 421. ISBN 978-1-84569-531-6. [Google Scholar]
- Gajovic, A.; Furic, K.; Tomasic, N.; Popovic, S.; Skokoc, Z.; Music, S. Mechanochemical preparation of nanocrystalline TiO2 powders and their behavior at high temperatures. J. Alloy. Compd. 2005, 398, 188–199. [Google Scholar] [CrossRef]
- Fan, X.; Sans, V.; Sharma, S.K.; Plucinski, P.K.; Zaikovskii, V.A.; Wilson, K.; Tennison, S.R.; Kozynchenko, A.; Lapkin, A.A. Pd/C catalysts based on synthetic carbons with bi- and tri-modal pore-size distribution: Applications in flow chemistry. Catal. Sci. Technol. 2016, 6, 2387–2395. [Google Scholar] [CrossRef] [Green Version]
- Siamaki, A.R.; Lin, Y.; Woodberry, K.; Connell, J.W.; Gupton, B.F. Palladium nanoparticles supported on carbon nanotubes from solventless preparations: Versatile catalysts for ligand-free Suzuki cross coupling reactions. J. Mater. Chem. A 2013, 1, 12909–12918. [Google Scholar] [CrossRef]
- Guerrero-Ortega, L.P.A.; Ramirez-Meneses, E.; Cabrera-Sierra, R.; Palacios-Romero, L.M.; Philippot, K.; Santiago-Ramirez, C.R.; Lartundo-Rojas, L.; Manzo-Robledo, A. Pd and Pd@PdO core–shell nanoparticles supported on Vulcan carbon XC-72R: Comparison of electroactivity for methanol electro-oxidation reaction. J. Mater. Sci. 2019, 54, 13694–13714. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Coloma, F.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. Effect of the presence of chlorine in bimetallic PtZn/CeO2 catalysts for the vapor-phase hydrogenation of crotonaldehyde. Appl. Catal. A-Gen. 2006, 304, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Abdelouahab-Reddam, Z.; Mail, R.E.; Coloma, F.; Sepúlveda-Escribano, A. Platinum supported on highly-dispersed ceria on activated carbon for the total oxidation of VOCs. Appl. Catal. A-Gen. 2015, 494, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure. Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Reinoso, F.R.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure. Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Jena, H.M.; Kumar, A. Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results. Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the composites are not available from the authors. |








| Metal Precursor | Pd(OAc)2 | K2PtCl4 | |||
| Support (100 mg) | 11 mg | 22 mg | 11 mg | 22 mg | |
| AC | AC-Pd1 | AC-Pd2 | AC-Pt1 | AC-Pt2 | |
| MWCNTs | CNT-Pd1 | CNT-Pd2 | CNT-Pt1 | CNT-Pt2 | |
| GO | GO-Pd2 | ||||
| Sample | Pd(wt%) | Pt(wt%) | ||
|---|---|---|---|---|
| Theoretical | Found (ICP-MS) | Theoretical | Found (ICP-MS) | |
| AC-Pd1 | 4.7 | 4.13 | - | - |
| AC-Pd2 | 8.5 | 7.63 | - | - |
| AC-Pt1 | - | - | 4.6 | 4.47 |
| AC-Pt2 | - | - | 8.5 | 7.28 |
| Sample | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| AC | 775 | 0.39 | 6.2 |
| AC-Pd1 | 747 | 0.42 | 6.9 |
| AC-Pd2 | 648 | 0.41 | 7.1 |
| AC-Pt1 | 722 | 0.40 | 6.9 |
| AC-Pt2 | 584 | 0.35 | 7.0 |
| Entry | Catalyst | Solvent | Base | Time (min) | Yield (%) b | TONc | TOF (h−1) d |
|---|---|---|---|---|---|---|---|
| 1 | AC | EtOH | K2CO3 | 30 | 0 | 0 | 0 |
| 2 | AC-Pd1 | EtOH | K2CO3 | 10 | 27 | 139 | 835 |
| 3 | EtOH | K2CO3 | 30 | 30 | 155 | 309 | |
| 4 | - | K2CO3 | 30 | 36 | 186 | 372 | |
| 5 | AC-Pd2 | EtOH | K2CO3 | 10 | 41 | 114 | 683 |
| 6 | EtOH | K2CO3 | 30 | 49 | 136 | 272 | |
| 7 | EtOH | K2CO3 | 180 | 69 | 192 | 64 | |
| 8 e | EtOH | Na2CO3 | 30 | 31 | 86 | 172 | |
| 9 e | EtOH | NaF | 30 | 2 | 6 | 12 | |
| 10 e | EtOH | KF | 30 | 34 | 94 | 189 | |
| 11 e | EtOH | Cs2CO3 | 30 | 35 | 97 | 194 | |
| 12 f | DCM | K2CO3 | 30 | 3 | 8 | 17 | |
| 13 f | ACN | K2CO3 | 30 | 5 | 14 | 28 | |
| 14 f | DMSO | K2CO3 | 30 | 4 | 11 | 22 | |
| 15 f | H2O | K2CO3 | 30 | 1.6 | 4 | 9 | |
| 16 | AC-Pt1 | EtOH | K2CO3 | 10 | 26 | 228 | 1.4 × 103 |
| 17 | EtOH | K2CO3 | 30 | 5 | 44 | 88 | |
| 18 | AC-Pt2 | EtOH | K2CO3 | 10 | 2.5 | 13 | 81 |
| 19 | EtOH | K2CO3 | 30 | 2.3 | 12 | 25 | |
| 20 | GO-Pd2 | EtOH | K2CO3 | 30 | 53 | - | - |
| 21 | CNT-Pd1 | EtOH | K2CO3 | 10 | 37 | - | - |
| 22 | CNT-Pd1 | EtOH | K2CO3 | 30 | 29 | - | - |
| 23 | CNT-Pd2 | EtOH | K2CO3 | 10 | 32 | - | - |
| 24 | CNT-Pd2 | EtOH | K2CO3 | 30 | 50 | - | - |
| 25 | CaCO3_5%Pd | EtOH | K2CO3 | 30 | 12 | - | - |
| 26 | Pd(OAc)2 | EtOH | K2CO3 | 30 | 75 | 34 | 68 |
| 27 | K2PtCl4 | EtOH | K2CO3 | 30 | 3 | 2.5 | 5 |
| Entry | Catalyst | Solvent | Base | Additive | Time (min) | Yield b | TON c | TOF (h−1) d |
|---|---|---|---|---|---|---|---|---|
| 1 | AC-Pd2 | EtOH | K2CO3 | Cyclooctene | 30 | 59 | 164 | 328 |
| 2 | - | Cyclooctene | 30 | 80 | 222 | 444 | ||
| 3 | EtOH | Cyclooctene | 180 | 38 | 106 | 35 | ||
| 4 | EtOH | Cyclohexene | 30 | 19 | 53 | 106 | ||
| 5 | EtOH | 1,5-COD | 30 | 21 | 58 | 117 | ||
| 6 | - | 1,5-COD | 30 | 39 | 108 | 217 | ||
| 7 | - | EtOH | 30 | 40 | 111 | 222 | ||
| 8 e | - | NaCl | 30 | 27 | 75 | 150 |
| Entry | Catalyst | Solvent | Base | Temperature (°C) | Time (min) | Yield (%) b | TON c | TOF (h−1) d |
|---|---|---|---|---|---|---|---|---|
| CH | ||||||||
| 1 | AC-Pd2 | EtOH | K2CO3 | 75 | 30 | 100 | 278 | 556 |
| 2 | 60 | 64 | 178 | 178 | ||||
| 3 | 360 | 65 | 181 | 30 | ||||
| 4 | 35 | 30 | 100 | 278 | 556 | |||
| 5 | 60 | 100 | 278 | 278 | ||||
| 6 | 360 | 50 | 139 | 23 | ||||
| MW | ||||||||
| 7 | AC-Pd2 | EtOH | K2CO3 | 75 | 30 | 61 | 169 | 339 |
| 8 | 60 | 100 | 278 | 278 | ||||
| 9 | 360 | 69 | 192 | 32 | ||||
| 10 | 35 | 30 | 52 | 144 | 289 | |||
| 11 | 60 | 56 | 156 | 156 | ||||
| 12 | 360 | 47 | 131 | 22 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M.A. Soliman, M.; F. Peixoto, A.; P.C. Ribeiro, A.; Kopylovich, M.N.; C.B.A. Alegria, E.; Pombeiro, A.J.L. Mechanochemical Preparation of Pd(II) and Pt(II) Composites with Carbonaceous Materials and Their Application in the Suzuki-Miyaura Reaction at Several Energy Inputs. Molecules 2020, 25, 2951. https://doi.org/10.3390/molecules25122951
M.A. Soliman M, F. Peixoto A, P.C. Ribeiro A, Kopylovich MN, C.B.A. Alegria E, Pombeiro AJL. Mechanochemical Preparation of Pd(II) and Pt(II) Composites with Carbonaceous Materials and Their Application in the Suzuki-Miyaura Reaction at Several Energy Inputs. Molecules. 2020; 25(12):2951. https://doi.org/10.3390/molecules25122951
Chicago/Turabian StyleM.A. Soliman, Mohamed, Andreia F. Peixoto, Ana P.C. Ribeiro, Maximilian N. Kopylovich, Elisabete C.B.A. Alegria, and Armando J.L. Pombeiro. 2020. "Mechanochemical Preparation of Pd(II) and Pt(II) Composites with Carbonaceous Materials and Their Application in the Suzuki-Miyaura Reaction at Several Energy Inputs" Molecules 25, no. 12: 2951. https://doi.org/10.3390/molecules25122951
APA StyleM.A. Soliman, M., F. Peixoto, A., P.C. Ribeiro, A., Kopylovich, M. N., C.B.A. Alegria, E., & Pombeiro, A. J. L. (2020). Mechanochemical Preparation of Pd(II) and Pt(II) Composites with Carbonaceous Materials and Their Application in the Suzuki-Miyaura Reaction at Several Energy Inputs. Molecules, 25(12), 2951. https://doi.org/10.3390/molecules25122951