2. Features of Synthesis of Vinyl Monomers from Plant Oils
Tang et al. [
16] reported on the synthesis of
N-hydroxyalkylamides and methacrylate hydrophobic monomers through the interaction of plant oil triglycerides with amino alcohols (
Figure 1). They developed a new interesting approach that involves the use of a two-step process. In the first stage, the interaction of triglycerides with amino alcohols with the formation of
N-hydroxyalkylamides by the amidation reaction:
Aminoalcohols, namely ethanolamine (R = H, n = 1), 3-amino-1-propanol (R = H, n = 2), and N-methylethanolamine (R = CH3, n = 1), were used as chemicals which enables the synthesis of a wide range of new monomers. In the second stage, the corresponding monomers were synthesized by the interaction between N-hydroxyethylamides R’–C(O)–N(R)–(CH2)n–CH2OH and methacrylic anhydride. In such a way, methacrylate monomer CH2=C(CH3)–C(O)–O–CH2–(CH2)n–N(R)–C(O)– (CH2)7–CH=CH–(CH2)7–CH3 based on high oleic soybean oil was synthesized.
A study of free radical polymerization of these monomers revealed that the double bonds in the fatty acid chains remain unaffected during the reaction. The synthesized methacrylate monomers based on high oleic soybean oil, depending on the nature of the branches in the side chain, have a wide range of glass transition temperatures [
16]. In further studies, Tang and co-authors used more than twenty amino alcohols to establish the mechanism of amidation of plant oils, in particular high-oleic soybean oil [
8]. Depending on the nature of the acyl fatty acid and amide structures, the polymers have a wide range of glass transition temperatures – from viscoelastic to thermoplastic materials. The possibility of controlled hydrogenation of unsaturated double bonds was also shown, which provides control over thermal and mechanical properties of polymers [
8].
On the other hand, Harrison and Wheeler have shown for the first time [
17] that the polymerization rate decreases with increasing unsaturation degree of acrylates containing acyl moieties of unsaturated fatty acids. They explained that the retarding of the polymerization rate and the low conversion are due to the interaction of radicals with the mobile hydrogen atoms in the acyl moieties. This leads to the formation of new allylic radicals of low activity which retards propagation of polymer chains (chain transfer reaction, retardation of the process) [
17].
Through the esterification reaction of fatty alcohols with acryloyl chloride, Chen and Bufkin synthesized a range of acrylates, including linoleyl acrylate, oleyl acrylate, and lauryl acrylate, and studied their copolymerizability. They showed that the presence of fatty fragments in acrylate molecules determines their polarity, which leads to alternating copolymerization with methyl methacrylate [
18].
In their next study [
19], Chen and Bufkin confirmed Harrison’s research results [
17] and showed that the presence of fatty acid moieties in the structure of the new acrylates leads to a decrease in both the polymerization rate and polymer molecular weights because of chain transfer reactions. Increasing the content of unsaturated acyl groups in the copolymer also reduces its glass transition temperature. The authors suggested the mechanisms for crosslinking acrylate copolymers and studied their physical and mechanical properties [
19].
Through amidation of triglycerides with amino alcohols, natural derivatives of surfactants and lubricants were obtained [
20]. It was demonstrated that the high reactivity of the vinyl group of the acrylate monomers based on plant oils determines the course of their polymerization and copolymerization by a free radical mechanism [
21]. Caillol et al. [
22,
23] proposed methods for the synthesis of a new monomer from cardanol (
Figure 2), which is isolated from cashew nuts. Reactivity of the cardanol-based monomer in the copolymerization reactions with the POBMs was studied [
24].
Cardanol-based polymers have a controlled flexibility due to “internal plasticization”, which occurs due to the presence of a long side chain. The presence of these fragments also provides polymeric materials based on cardanol with hydrophobic properties. It should be noted that cardanol exhibits antimicrobial and anti-thermite properties and, under certain conditions, can provide them to copolymers based on it. It is compatible with a wide range of polymers, such as alkyds, melamines, polyesters, etc. [
22].
In the study [
23], synthetic pathways to molecular blocks from cardanol by one- or two-stage methods were reported. In particular: 1) dimerization/oligomerization of cardanol, to increase its functionality; 2) synthesis of reactive amines by thiolene coupling; 3) epoxidation and (meth)acrylation of cardanol for the synthesis of polymers and materials with oxirane or (meth)acrylate groups.
A feature of cardanol-based monomers and polymers is the presence of aromatic fragments and fatty moieties in their structure at the same time. The authors showed that cardanol-based polymeric materials have prospective properties for manufacturing coatings, in particular, with enhanced mechanical and thermal properties [
23].
Recently, methacrylated ricinoleic acid monomer (
Figure 3) was synthesized by the esterification reaction of ricinoleic acid with 2-hydroxyethyl methacrylate [
25]:
Other plant oil-based methacrylates have been widely studied in atom transfer radical polymerization (ATRP). A method for the ATRP of lauryl methacrylate was developed by Mandal et al. to obtain narrowly distributed polymers with predefined molecular weights [
26]. Fatty alcohol derived methacrylates were used to prepare well defined main chain substituted polymethacrylates by copper-mediated ATRP [
27].
It should be noted that all known methods for the synthesis of monomers from plant oil triglycerides are multi-stage and involve several steps, which makes their implementation in industry more challenging.
Recently, we developed a one-step process for the synthesis of new vinyl monomers by the direct transesterification reaction of plant oil triglycerides with
N-(hydroxyethyl)acrylamide (
Figure 4) [
13,
28].
Using this process, a range of POBMs was synthesized [
10,
12]. In the reaction,
N-(hydroxyethyl)acrylamide can be considered as an alcohol ROH – where the unsaturated fragment CH
2=CH–C(O)–NH–CH
2–CH
2– is a residue –R. Upon the alcoholysis (transesterification), a residue exchange between the triglyceride and
N-(hydroxyethyl)acrylamide occurs leading to formation of the corresponding monomers.
The POBMs synthesis was conducted in THF with an excess of N-(hydroxyethyl)acrylamide (molar ratio of N-(hydroxyethyl)acrylamide to triglyceride as 5.9: 1) in order to achieve complete transesterification of the triglycerides. The yield of the desired monomers was about 94–96%. The reaction by-product (glycerol) and the excessive N-(hydroxyethyl)acrylamide are easily removed by washing with brine after diluting the reaction mixtures with CH2Cl2. The POBMs sparingly soluble in water remain in the organic phase. To avoid free radical polymerization, 2,6-di-tert-butyl-p-cresol (0.05 to 0.1% by weight) was added as an inhibitor to the reaction mixtures prior the synthesis.
Upon the alcoholysis of olive oil with N-(hydroxyethyl)acrylamide, 2-N-acryloylaminoethyl oleate CH2=CH–C(O)–NH–CH2CH2–O–C(O)–(CH2)7–CH=CH–(CH2)7–CH3 is predominantly formed.
This monomer contains one double bond in the acryloylamide moiety and one double bond in the fatty acid chain. Such a structure, in comparison with the soybean oil-based monomer 2-N-acryloylaminoethyl linoleate (CH2=CH–C(O)–NH–CH2CH2–O–C(O)–(CH2)7–CH=CH–CH2– CH=CH–CH2–(CH2)3–CH3) reduces the extent of the chain transfer reaction. Accordingly, fewer less active radicals are formed and the polymerization reaction proceeds to higher conversion with a less pronounced retardation effect. This leads to the formation of polymers (copolymers) with a higher molecular weight and a lower polydispersity index.
The composition of plant oils is known to depend on the type of crop raw material. Therefore, an important step was to determine the content of different fatty acid chains in the mixture of monomers obtained upon the transesterification of olive oil with
N-(hydroxyethyl)acrylamide. The content of fatty acid chains in the oil was determined from the integral intensities of the characteristic proton signals of each fatty acid chain and glycerol fragments using
1H-NMR spectroscopy of the plant oil [
29] (
Figure 5).
The content of the linolenic acid units (linolenate) in olive oil was calculated by measuring the integral value of the signal at 1 ppm, which corresponds to the protons of the methyl group in a linolenic acid chain (signal E in
Figure 5). Taking into account only one of the signals of α-protons of glycerol, i.e., the signal at 4.27 ppm (
Figure 5), the ratio of the integral values is two α-glycerol protons to three protons of the linolenic acid methyl group. It should be kept in mind that three linolenic acid chains can undergo transesterification in the same glycerol molecule. A field correction factor is the ratio of two protons of glycerol to nine protons of the linolenic acid methyl groups. By converting the ratio of these areas to percentages, a ratio of 22.2% glycerol to 100% linolenic acid was obtained [
29]. Calibration of the integral value of one of the glycerol α-proton signals to the value in the
1H-NMR spectrum in the signal region at 0.98 ppm directly gives the percentage of linolenic acid in the sample −0.73%.
The percentage of linoleic acid chains (linoleates) in olive oil was determined from the ratio of the integral value of the signal at ~2.74 ppm, which corresponds to the methylene protons between two double bonds (signal A in
Figure 5), to the integral value of one of glycerol α-protons. The field correction factor (33.3) is the ratio of two glycerol protons to six possible methylene protons in linoleate. The amount of linoleic acid is calculated by subtracting the twiced content of linolenic acid, determined from the peak at 2.74 ppm [
26].
The percentage of oleic acid chains (oleates) was determined from the ratio of the integral value of the signal at ~2.02 ppm, which corresponds to the protons in the α-position to the double bond of all unsaturated fatty acids (signal C in
Figure 5), to the integral value of one of glycerol α-protons. Accordingly, the ratio of two glycerol protons to twelve possible protons in the α-position to the double bond of all unsaturated fatty acids is the field correction factor equal to 16.7. The percentage of oleic acid was calculated by subtracting the content of unsaturated linolenic and linoleic acid chains from the determined value [
29].
The content of saturated fatty acid chains was determined from the fact that the total content of fatty acids is 100%, and the amount of unsaturated fatty acid chains was subtracted from 100%. The determined content of fatty acid chains is given in
Table 1. These data are in a good agreement with the literature data obtained by gas chromatography of olive oil [
30].
Thus, according to 1H-NMR spectroscopy, the olive oil triglycerides include: saturated fatty acid chains (C18:0) −12.44%; oleic acid chains (C18:1) −80.23%; linoleic acid chains (C18:2) −6.60%; and linolenic acid chains (C18:3) −0.73%.
These results are consistent with the literature data obtained by gas chromatography of olive oil, where the saturated chains make up 1–20%, oleic acid chains 55–83%, linoleic acid chains 3.5–21%, and linolenic acid chains less than 1% [
31]. Using this approach, monomers based on various plant oils were successfully synthesized and characterized (
Table 2).
The monomer structure was confirmed by FTIR and
1H-NMR spectroscopy [
12]. A
1H-NMR spectrum of the olive oil-based monomer is shown in
Figure 6. Similar spectra are recorded for other POBMs, which confirms the presence of the acryloylamide moiety in their molecules. This allows for predicting similar reactivity of the vinyl group in the monomers acryloylamide moiety in the radical polymerization [
12].
The FTIR spectroscopy data indicate the addition of fatty acid acyl moieties to the acrylamide fragment (e.g., for the olive monomer—
Figure 7). FTIR spectra of monomers based on other plant oils are pretty similar. Hence, the synthesized POBMs can be attributed to the conventional vinyl monomers because the acryloylamide moiety provides the participation of these monomers in the free radical polymerization.
The content of fatty acid chains in the monomer mixture produced by the transesterification of plant oils with
N-(hydroxyethyl)acrylamide was determined on the example of the olive monomer by calculating the ratio of the integral values in the
1H-NMR spectra of the commercial oil and the resulting monomer mixture (
Figure 8).
According to the 1H-NMR spectroscopy data, the monomer mixture consists of: saturated fatty acid chains (C18:0) −11.47% (stearic and palmitic); oleic acid chains (C18:1) −81.9%; linoleic acid chains (C18:2) −5.9%; and linolenic acid chains (C18:3) −0.73% after the olive oil transesterification.
Thus, the major component of the olive monomer is a monomer with oleic acid chains (81.9%) – 2-N-acryloylaminoethyl oleate. The soybean monomer is mainly a monomer with linoleic acid chains (57.5%) – 2-N-acryloylaminoethyl linoleate whereas the linseed monomer predominantly consists of a monomer with linolenic acid chains (58%) – 2-N-acryloylaminoethyl linolenate.
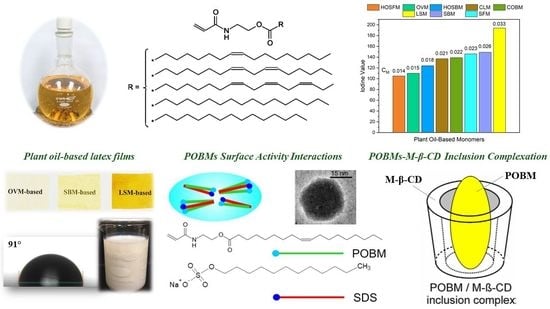
One of the most important characteristics of monomers is the degree of unsaturation, which for POBMs is determined by the number of the double bonds in the fatty acid chains. To compare the monomers in terms of the unsaturation degree, their iodine values were determined and compared with those for the corresponding plant oils used for the synthesis of the POBMs (
Table 2). The obtained results show that the iodine value for the monomers is higher than those for the oils, due to the presence of an unsaturated acryloylamide moiety. Depending on the type of oil, the iodine values for various monomers differ. For instance, the iodine value for 2-
N-acryloylaminoethyl oleate (110 g/100 g) is significantly lower than that for 2-
N-acryloylaminoethyl linoleate (149 g/100 g) due to the different unsaturation degree of the molecules. Besides the acryloylamide moiety, there are two double bonds in the fatty acid chain of the soybean monomer molecule [
10]. The low solubility of these monomers in water implies their highly hydrophobic nature [
12].
Analysis of the data obtained from the synthesis and characterization of plant oil-based acrylic monomers allows for drawing the POBM general formula (
Figure 9) as:
3. Features of Homo- and Copolymerization of Plant Oil-Based Acrylic Monomers
The reactivity of the POBMs in the free radical polymerization reactions (chain growth reaction) is determined by the presence of an acryloylamide moiety containing a vinyl group which is common for all monomers. However, the POBMs contain a certain amount of unsaturated fatty acid chains of various structures (the different numbers of both double bonds and hydrogen atoms in the α-position to the double bonds). This causes the monomers to participate in chain transfer reactions due to the abstraction of the allylic hydrogen atoms and the formation of less active radicals. Hence, the monomer chain transfer constants C
M clearly depend on monomer structure as follows: 0.033 (most unsaturated LSM) > 0.026 (SBM) > 0.023 (SFM) > 0.015 (least unsaturated OVM) with respect to decreasing number of C−H groups in the α-position of the fatty acid double bonds) [
12]. This impacts the polymerization conversion and molecular weights of the resulting polymers (copolymers) from the POBMs.
The features of homopolymerization kinetics for 2-
N-acryloylaminoethyl oleate revealed that the orders of reaction with respect to monomer and initiator are 1.06 and 1.2, respectively. The unsaturation degree of fatty acid chains in the POBM molecules was used as a criterion for comparing the kinetic data of homopolymerization of 2-
N-acryloylaminoethyl oleate and 2-
N-acryloylaminoethyl linoleate. The observed deviations of the orders of reaction are due to the specific mechanism of the homopolymerization reaction of 2-
N-acryloylaminoethyl oleate which includes two simultaneous reactions (chain propagation and transfer) [
12]. Although the propagating radicals might be very reactive, once the chain is transferred to the allylic C−H, the newly formed radical becomes more stable due to resonance stabilization and does not readily initiate new chains. In comparison with the soybean monomer, the olive monomer is less involved into the chain transfer reactions (the chain transfer constant C
M = 0.015 for OVM while for SBM C
M = 0.026). As a result, the homopolymerization of the olive monomer occurs at a higher rate of 12.2–45.3 · 10
−5 mol/(L·s) when compared with the more unsaturated soybean monomer −4.3−11.3·10
−5 mol/(L·s). The molecular weights of the homopolymers were determined by gel permeation chromatography. The resulting homopolymers from OVM have higher number average molecular weights (16 800–23 200 g/mol for 2-
N-acryloylaminoethyl oleate compared to 13 600–14 300 g/mol for 2-
N-acryloylaminoethyl linoleate).
The reactivity of the POBMs in chain copolymerization was studied in their reactions with styrene and vinyl acetate. A characteristic feature of the POBMs is the presence of the acryloylamide fragment (CH
2=CH–C(O)–NH–CH
2–CH
2–) in their structure, which determines the monomer reactivity in copolymerization. The composition of the copolymers was determined from
1H-NMR spectroscopy data. Radical copolymerization of the plant oil-based monomers is described with the classical Mayo–Lewis equation. The Alfrey−Price Q (0.41–0.51) and e (0.09–0.28) parameters are close for all POBMs and do not essentially depend on the monomer structure [
13]. This is due to the presence of the same acryloylamide moiety in the POBM molecules which determines the monomer reactivity in polymerization reactions.
The features of copolymerization are determined by the structure of monomers based on plant oil triglycerides along with the degradative chain transfer by formation of less active radicals. Nevertheless, the growth of macrochains can be described with the conventional features of chain copolymerization, yielding copolymers with a potential to be used in a broad variety of applications.
Vinyl monomers from plant oils that have different degrees of unsaturation, soybean, and olive oils, were copolymerized in emulsion with styrene to investigate the kinetics features and feasibility of latex formation. The kinetics of emulsion copolymerization of styrene with the olive and soybean monomers agree with the Smith-Ewart theory since the number of nucleated latex particles is proportional to the surfactant and initiator concentration to the powers 0.58–0.64 and 0.39–0.46, respectively [
33]. Copolymerization of styrene with POBMs follows the typical phenomenology for emulsion polymerization of hydrophobic monomers with a micellar nucleation mechanism. When the POBMs were copolymerized in emulsion with significantly more water soluble comonomers, methyl methacrylate and vinyl acetate, latex particle nucleation mainly occurred through homogeneous mode. For the emulsion copolymerization of methyl methacrylate with OVM and SBM, the reaction orders with respect to emulsifier and initiator are 0.33–0.67 and 0.56–0.69, respectively. It was shown that upon adding the highly hydrophobic POBMs into the monomer mixture, the latex polymer particles originating from micellar nucleation essentially increases [
15].
Using emulsion and miniemulsion copolymerization of the olive and soybean monomers with styrene or methyl methacrylate, stable aqueous dispersions of polymers with latex particle sizes of 40–210 nm were produced. The content of the POBM units in the macromolecules of the latex polymers is 5–60 wt.%. The average molecular weight of the synthesized polymers varies in the range of 30,000–391,500. It was found that the molecular weight of the latex copolymers decreases with increasing unsaturation degree of the POBMs and their content in the reaction mixture, which is explained by degradative chain transfer to unsaturated fatty acid chains [
15,
33].
An analysis of the literature shows a rapid development of a new branch in polymer science related to the chemistry of aliphatic and aromatic biobased monomers obtained from renewable plant sources. The synthesis of such monomers allows for producing fully biobased polymers with biocompatible and biodegradable properties which do not pollute the environment. Copolymerization of monomers synthesized from various plant oils, which have different unsaturation, enables formation of copolymers with side branches of the macrochain with different unsaturation degrees. A large variety of the POBMs allows to synthesize polymers having moieties with different unsaturation and to make coatings with adjustable cross-linking degree thereof, including latex copolymers from fully biobased monomer mixtures [
24]. Remarkably, copolymerization of the POBMs with commercial vinyl monomers enables synthesis of polymers with enhanced hydrophobicity along with the mechanism of internal plasticization.
Synthesized latex polymers and copolymers are prospective candidates for the formation of moisture-/water-resistant polymer coatings with controlled physical and mechanical properties using controlled content of incorporated POBM units with different unsaturation in the latex structure.
4. Preparation of Polymeric Coatings from Plant Oil-Based Monomers
As shown by Solomon [
34], both crude and modified plant oils are common film-forming materials for the production of paints and varnishes. Despite the emergence of various synthetic polymers based on vinyl and acrylic monomers, crude plant oils are still the basis of some paints for painting the roofs of houses and other outdoor objects. One of the disadvantages of such materials is the slow drying and relatively low moisture resistance. The synthesis of plant oil-based acrylic monomers opens new avenues for producing homo- and copolymers thereof with adjustable physical and mechanical properties (e.g., flexibility, strength,
etc.) due to formation of three-dimensional networks with controlled cross-linking density. The development of such coatings was shown to be carried out through oxidative cross-linking of the polymer network [
35].
It should be noted that upon POBM application for the synthesis of latex polymers as highly hydrophobic film-forming protective coatings, they form three-dimensional networks with adjustable cross-linking density through the oxidative cross-linking mechanism.
Triglycerides of linseed oil contain about 52% of linolenic (
cis,
cis,
cis-9,12,15-octadecatrienoic) acid chains which have three isolated carbon-carbon double bonds in their structure (
Figure 10). Other fatty acids in linseed oil are oleic (
cis-9-octadecenoic, 22%) and linoleic (
cis,
cis-9,12-octadecadienoic, 16%) acids. Hence, linolenate is a major constituent of LSM.
Oxidative cross-linking of the LSM-based copolymers is a free radical chain process consisting of chain initiation, propagation, and termination steps. Initiation, i.e., the formation of a fatty acid chain radical, can occur by thermal homolytic cleavage of a C–H bond or by a hydrogen atom abstraction from C–H by an initiator free radical. The bond dissociation energy of a bisallylic hydrogen is approximately 42 kJ/mol lower than that of an allylic hydrogen. As a result, linoleates and linolenates are more readily autooxidized and cross-linked in comparison with oleates.
Hydrogen atom abstraction from C-11 or C-13 of the linolenate moiety leads to pentadienyl radicals I and II (
Figure 10). Subsequent oxygen addition to radicals I and II generates peroxyl radicals which are able of abstracting a hydrogen atom from a donor such as another linolenate moiety to form conjugated
trans,cis-hydroperoxides I–IV (
Figure 10). The hydroperoxides undergo decomposition reaction (
Figure 11). The last three reactions lead to cross-linking of the macromolecules and formation of a polymer network.
The main mechanical characteristics of films from latex polymers based on styrene or methyl methacrylate and the POBMs were determined. The composition of the copolymers was calculated from the
1H-NMR spectroscopy data. The content of the olive and soybean monomers in the reaction mixture varied from 10 to 40 wt. % for copolymers with methyl methacrylate and from 25 to 60 wt. % for copolymers with styrene. A plasticizing effect and enhanced hydrophobicity were observed for the copolymers synthesized from 2-
N-acryloylaminoethyl oleate and 2-
N-acryloylaminoethyl linoleate as comonomers. The unsaturation degree of the olive and soybean monomers was used as an experimental parameter to control the latex properties. The effect of monomer unsaturation on the cross-linking density of the latex films and, thus, on the physical and mechanical properties of the coatings was demonstrated [
35].
The decrease in the glass transition temperature of the latex copolymers (from 105 to 57 °C for the copolymers of the olive and soybean monomers with methyl methacrylate and from 100 to 5 °C for the copolymers with styrene) indicates that the presence of the OVM and SBM units in the macromolecules affects thermomechanical properties of the resulting latex copolymers. The olive and soybean monomer units impart flexibility to the macromolecules, improve the conditions of film formation, increase the strength in comparison with conventional polystyrene and poly(methyl methacrylate). Moreover, the OVM and SBM units in the macromolecules increase the hydrophobicity of polymeric latex films thus reducing the negative impact of water on the properties of the coatings.
Therefore, the incorporation of the hydrophobic fatty acid chains into the macromolecules of latex polymers allows for formation of polymer networks with a controlled cross-linking density along with enhancing the water resistance of the coatings. The copolymers based on the olive and soybean monomers enable the formation of coatings with low surface energy and water-repellent properties.
5. Formation of Micelles from the Complexes of Highly Hydrophobic Plant Oil-Based Monomers with Sodium Dodecyl Sulfate
The synthesized POBMs contain CH2=CH–C(O)–NH–CH2CH2–O–C(O)– as a hydrophilic fragment in their chemical structure and R– as a hydrophobic constituent in the fatty acid chain. Such a monomer structure imparts unique properties to the POBMs associated with the ability to participate in intermolecular (electrostatic and/or van der Waals) interactions to produce complexes (aggregates).
The influence of high oleic soybean oil-based (HO-SBM) and olive oil-based acrylic monomers on micellization of sodium dodecyl sulfate (SDS) was studied at different monomer and surfactant concentrations. The obtained data imply that SDS is able to solubilize highly hydrophobic monomer molecules. This leads to development of mixed (SDS/POBM) micelles (
Figure 12). Surface activity of a SDS/POBM mixture varies by adding the monomer and, as a rule, is higher than for the surfactant alone [
36]. It was also found that monomer molecules replace surfactant counterparts in the mixed micelles. Both the DLS and TEM data allow for conclusion that incorporation of POBM into the mixed micelles facilitates micellar association and development of 25–30 nm structures. Hence, solubilization of monomer molecules by SDS can affect mechanism of emulsion polymerization and reaction kinetics, as well as have an effect on development of latex particles and their morphology.
Surfactants form direct micelles due to the interactions between hydrocarbon parts of their molecules. Usually, the micelle has a layered structure depending on the packaging of surfactant molecules. A micelle contains a hydrocarbon nucleus, a water-hydrocarbon layer, which includes 2–3 methylene groups, and a layer of hydrated polar groups. It should be noted that surfactant molecules are freely localized in the micelle. They can leave the micelle or move to a different position; they can even be engulfed into the nucleus, which is determined by the hydrophobicity of the surfactant fragment [
37,
38].
The feature of micellization of sodium dodecyl sulfate (SDS) in the presence of the POBMs is surfactant ability to solubilize highly hydrophobic monomer molecules and promote development of mixed (SDS/POBM) micelles. As shown by Kingsley et al. [
36], the packing of surface-active SDS/POBM complexes (aggregates) and the SDS molecules occurs upon micellization. In this event, the SDS molecules can be squeezed out of the micelle and localized at the interphase reducing the interfacial tension. Sodium dodecyl sulfate is known to have a micelle aggregation number of 60–70 [
38]. It was found that the aggregation number decreases to 10–48 (
Table 3) upon micellization of the SDS/POBM complexes. The micellar size depends on the packaging of the surfactants. Their length and packing density in the micelle determine the radius of the hydrocarbon nucleus. The formed colloidal solution simultaneously contains the SDS micelles and the mixed SDS/POBM micelles. It should be noted that their size is 18–38 nm (
Table 3,
Figure 13) whereas the SDS micellar size is 1.7–3.1 nm.
If the fatty acid chain cannot leave the hydrocarbon nucleus, the polar group can be even drawn into the hydrophobic nucleus [
38], which also affects the aggregation number. The authors explain the features of micellization (micellar parameters, size, structure, and surface tension) and the obtained results with the formation of surface-active SDS/POBM complexes (aggregates) of the following structure (
Figure 14):
The formation of a water-hydrocarbon layer of the spherical micelle leads to the localization of the acrylic groups of the POBM molecule at the interface. This opens up new possibilities for the formation of “core–shell” morphology, providing ability of POBM to undergo polymerization.
Chemical structure of POBM and SDS molecules is similar. They both have hydrophilic “head” and long non-polar “tail” with in average 17 (POBM) and 12 (SDS) carbon atoms. It was suggested that physical association of both polar “heads” and hydrophobic “tails” in water underlie intermolecular interactions. The results indicate that surface activity of a SDS/POBM mixture is usually higher than for the surfactant and varies by adding the monomer. Moreover, structure, size, and micellar parameters, observed changes upon the addition of the POBMs. The formed complexes can open up new opportunities for micellization and, thus, new approaches to conducting heterophase polymerization (e.g., conventional emulsion and miniemulsion polymerization).
6. Dual Role of Methyl-β-Cyclodextrin in the Emulsion Polymerization of Highly Hydrophobic Plant Oil-Based Monomers
The new acrylic monomers are also capable of complexation with cyclodextrins, which play a dual role in both protecting against allylic termination chain transfer as well as improving monomer polymerizability.
Methyl-β-cyclodextrin (M-β-CD), amphiphilic oligosaccharide, was utilized to enhance the polymerizability of high oleic soybean oil-based and linseed oil-based monomers in copolymerization with styrene. Interactions between M-β-CD and the POBMs leading to the development of 1:1 “host-guest” complex were revealed. In the presence of the oligosaccharide, polymer yield rises whereas the coagulum content decreases during the emulsion polymerization of POBMs with styrene implying their improved solubility in water. Latex polymers with a higher molecular weight were synthesized in the presence of inclusion complexes. Incorporation of POBM molecules into the M-β-CD cavities protects the fatty acid chains and reduces chain transfer as confirmed by 1H-NMR spectroscopy. This effect is more pronounced for more unsaturated linseed oil-based monomer.
These results are explained by the specific structures of M-β-CD having a hydrophobic cavity and a hydrophilic exterior, and the POBM molecule, possessing a long hydrophobic fatty acid chain; this results in the formation of an inclusion complex (
Figure 15).
To confirm the formation of the inclusion complex, it was used Powder X-ray diffractometry (PXRD) [
39]. The PXRD spectra of H-SBM [monomer from hydrogenated (very low degree of unsaturation) soybean oil], M-β-CD, H-SBM/M-β-CD inclusion complex, and the physical mixture are depicted in
Figure 16A. The diffractogram of the physical mixture of M-β-CD and H-SBM was the superposition of the monomer and the oligosaccharide implying that there is no interaction between them [
40]. The diffraction pattern of the H-SBM/M-β-CD inclusion complex displayed no characteristic peaks that the pure monomer had. These data reveal that the self-lattice arrangement of the monomer was changed from a crystalline to amorphous state due to the H-SBM inclusion into the methyl-β-cyclodextrin cavity.
The diffractograms of HO-SBM, M-β-CD, and HO-SBM/M-β-CD inclusion complex are shown in
Figure 16B. As both M-β-CD and high oleic soybean oil-based monomer are non-crystalline substances, the development of HO-SBM/M-β-CD inclusion complex cannot be proven by PXRD.
To this end, differential scanning calorimetry [
41] was used to further confirm the complexation. The DSC thermograms of H-SBM, M-β-CD, H-SBM/M-β-CD inclusion complex, and the physical mixture are shown in
Figure 17. Although the endothermic peaks of the monomer were detected in the DSC curve of the physical mixture, no peak in the melting range of the monomer was detected in the thermogram of H-SBM/M-β-CD. This confirms that the H-SBM molecules are entirely included into the oligosaccharide cavities and H-SBM/M-β-CD inclusion complex forms.
No peak in the melting range of high oleic soybean oil-based monomer was observed in the DSC curves of HO-SBM/M-β-CD inclusion complex and their physical mixture (data not shown). Obviously, this is because the HO-SBM molecules might be able to penetrate into the M-β-CD cavities since the monomer is an oily liquid. This phenomenon is known as the kneading method for the formation of cyclodextrin-guest complexes [
42].
Even though PXRD and DSC studies confirmed the transitions from a crystalline to amorphous state of H-SBM upon its interaction with M-β-CD, these analyses neither definitely confirmed inclusion complexation (especially, for HO-SBM) nor determined the complex stoichiometry. Thus, electrospray ionization–mass spectrometry (ESI–MS) [
42,
43] was used to investigate the complexation of M-β-CD with POBM.
The ESI mass spectra of inclusion complexes of the monomers with M-β-CD are presented in
Figure 18. Ions detected in the spectrum of H-SBM/M-β-CD (
Figure 18A) are attributed to sodium adducts of complexes of stearate-H-SBM with M-β-CD having 9–13 methyl groups. In the ESI mass spectrum of the HO-SBM/M–β–CD inclusion complex (
Figure 18B), ions that correspond to the inclusion of oleate-HO-SBM by M
10-13-β-CD (sodium adducts) are revealed.
Thus, the M-β-CD complexation of monomer molecules leading to the 1:1 complex formation (
Figure 15) was directly confirmed by the ESI–MS data. The emulsion copolymerization of the POBMs with styrene in the presence of methyl-β-cyclodextrin was found to allow for the preservation and protection of fatty acid double bonds (–CH=CH–) and the bisallylic hydrogen atoms (–CH=CH–CH
2–CH=CH–). The inclusion complexation decreases the chain transfer contribution by protecting the allylic moiety of the monomer fatty acid chains and simultaneously improves the aqueous solubility and availability of the monomers in the emulsion polymerization process. Therefore, a higher molecular weight of latex polymers from the highly hydrophobic POBMs was achieved with higher monomer conversion and lower coagulum formation.