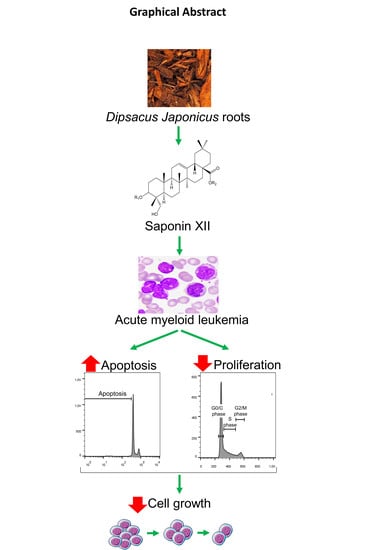
Cell Growth Inhibition of Saponin XII from Dipsacus japonicus Miq. on Acute Myeloid Leukemia Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of Saponin XII
2.2. Biological Activity of Isolated Saponin XII
3. Materials and Methods
3.1. Chemical Methods
3.2. Extraction and Purification of Saponin XII
3.3. Structural Characterizations of Saponin XII (1)
3.4. OCI-AML Culture Conditions
3.5. Analysis of Cell Number, Apoptotic Cell Death and Cell Cycle Progression
3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adorisio, S.; Fierabracci, A.; Rossetto, A.; Muscari, I.; Nardicchi, V.; Liberati, A.M.; Riccardi, C.; Van Sung, T.; Thuy, T.T.; Delfino, D.V. Integration of Traditional and Western Medicine in Vietnamese Populations: A Review of Health Perceptions and Therapies. Nat. Prod. Commun. 2016, 11, 1409–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.-M.; Shi, Y.-P. Phytochemicals and Biological Activities of Dipsacus Species. Chem. Biodivers. 2011, 8, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Ma, L.; Barrie, F.R. Flora of China Editorial Board; Fl. Reipubl. Popularis Sin.; Science Press: Beijing, China, 1986; Volume 658, pp. 19, 654. [Google Scholar]
- Loi, D.T. Glossary of Vietnamese Medical Plants; Science and Techniques Publishing House: Hanoi, Vietnam, 2000; p. 759. [Google Scholar]
- Liu, Z.L.; Jiang, G.H.; Zhou, L.; Liu, Q.Z. Analysis of the Essential Oil of Dipsacus japonicus Flowering Aerial Parts and its Insecticidal Activity against Sitophilus zeamais and Tribolium castaneum. Z. Nat. C 2013, 68, 13. [Google Scholar] [CrossRef]
- Wei, F.; Lou, Z.C.; Gao, M.; Miao, Z.C. Application of new techniques of NMR in structure elucidation of japondipsaponin E1 isolated form Dipsacus japonicus Miq. Acta Pharm. Sin. 1995, 30, 831–837. [Google Scholar]
- Wei, F.; Liu, L.-M.; Lou, Z.-C. Application of new techniques of NMR in structure elucidation of japondipsaponin E2 isolated form Dipsacus japonicas Miq. J. Shenyang Pharm. Univ. 1998, 15, 120–124. [Google Scholar]
- Thuy, T.T.; Sung, T.V.; Adam, G. Study on the chemical composition of the Dipsacus japonicus. Part. I. Iridoid and bisiridoid glycosides. Vietnam J. Chem. 1999, 37, 64–69. [Google Scholar]
- Thuy, T.T.; Sung, T.V.; Adam, G. Study on the chemical composition of the Dipsacus japonicus. Part. II. Triterpene glycosides. Vietnam J. Chem. 2002, 40, 13–19. [Google Scholar]
- Miao, Z.-C.; Zhou, Y.-X.; Wei, F. Chemical structure and NMR of a new saponin from Dipsacus japonicus (Dipsacaceae). Acta Bot. Sin. 2000, 42, 421–426. [Google Scholar]
- Miao, Z.-C.; Zhou, Y.-X.; Feng, R.; Wei, F. Structural determination of a new bidesmosidic triterpenoid glycoside from Dipsacus japonicus. Chin. J. Org. 2000, 20, 81–87. [Google Scholar]
- Ji, D.; Wu, Y.; Zhang, B.; Zhang, C.-F.; Yang, Z. Triterpene saponins from the roots of Dipsacus asper and their protective effects against the Aβ25–35 induced cytotoxicity in PC12 cells. Fitoterapia 2012, 83, 843–848. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Ndontsa, B.L.; Kuete, V.; Nguekeu, Y.M.; Çelik, I.; Mbouangouere, R.; Tane, P.; Efferth, T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 2018, 43, 78–85. [Google Scholar] [CrossRef]
- Yu, J.-H.; Yu, Z.-P.; Wang, Y.-Y.; Bao, J.; Zhu, K.-K.; Yuan, T.; Zhang, H. Triterpenoids and triterpenoid saponins from Dipsacus asper and their cytotoxic and antibacterial activities. Phytochemistry 2019, 162, 241–249. [Google Scholar] [CrossRef]
- Kauno, I.; Tsuboi, A.; Nanri, M.; Kawano, N. Acylated triterpene glycoside from roots of Dipsacus asper. Phytochemistry 1990, 29, 338–339. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Xue, Z. Structure determination of saponin XI, XII and XIII from Dipsacus asper Wall. Acta Pharm. Sin. 1993, 28, 358–363. [Google Scholar]
- Thuy, T.T.; Huong, N.T.T.; Nhung, L.T.H.; Ninh, P.T.; Delfino, D.V.; Van Sung, T. Isolation, characterisation and biological evaluation of a phenoxazine, a natural dyestuff isolated from leaves of Peristrophe bivalvis. Nat. Prod. Res. 2013, 27, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Delfino, D.V.; Pozzesi, N.; Pierangeli, S.; Ayroldi, E.; Fierabracci, A. Manipulating thymic apoptosis for future therapy of autoimmune diseases. Curr. Pharm. Des. 2011, 17, 3108–3119. [Google Scholar] [CrossRef]
- Cao, H.; Li, C.; Qi, W.; Meng, X.; Tian, R.; Qi, Y.; Yang, W.; Li, J. Synthesis, cytotoxicity and antitumour mechanism investigations of polyoxometalate doped silica nanospheres on breast cancer MCF-7 cells. PLoS ONE 2017, 12, e0181018. [Google Scholar] [CrossRef] [Green Version]
- Noté, O.P.; Kamto, E.L.D.; Toukea, D.D.; Aouazou, S.A.; Mbing, J.N.; Muller, C.D.; Guillaume, D.; Pegnyemb, D. Pro-apoptotic activity of new triterpenoid saponins from the roots of Albizia adianthifolia (Schumach.) W.Wight. Fitoterapia 2018, 129, 34–41. [Google Scholar] [CrossRef]
- Li, X.-R.; Li, J.; Sun, S. The molecular mechanism of treating osteoarthritis with dipsacus saponins by inhibiting chonderocyte apoptosis. Exp. Ther. Med. 2017, 14, 4527–4532. [Google Scholar]
- Adorisio, S.; Fierabracci, A.; Muscari, I.; Liberati, A.M.; Cannarile, L.; Thuy, T.T.; Van Sung, T.; Sohrab, H.; Hasan, C.M.; Ayroldi, E.; et al. Fusarubin and Anhydrofusarubin Isolated from A Cladosporium Species Inhibit Cell Growth in Human Cancer Cell Lines. Toxins 2019, 11, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cham, B.T.; Linh, N.T.T.; Thao, D.T.; Anh, N.T.H.; Tam, N.T.; Anh, B.K.; Muscari, I.; Adorisio, S.; Sung, T.V.; Thuy, T.T.; et al. Cell Growth Inhibition of Saponin XII from Dipsacus japonicus Miq. on Acute Myeloid Leukemia Cells. Molecules 2020, 25, 3325. https://doi.org/10.3390/molecules25153325
Cham BT, Linh NTT, Thao DT, Anh NTH, Tam NT, Anh BK, Muscari I, Adorisio S, Sung TV, Thuy TT, et al. Cell Growth Inhibition of Saponin XII from Dipsacus japonicus Miq. on Acute Myeloid Leukemia Cells. Molecules. 2020; 25(15):3325. https://doi.org/10.3390/molecules25153325
Chicago/Turabian StyleCham, Ba Thi, Nguyen Thi Thuy Linh, Do Thi Thao, Nguyen Thi Hoang Anh, Nguyen Thanh Tam, Bui Kim Anh, Isabella Muscari, Sabrina Adorisio, Tran Van Sung, Trinh Thi Thuy, and et al. 2020. "Cell Growth Inhibition of Saponin XII from Dipsacus japonicus Miq. on Acute Myeloid Leukemia Cells" Molecules 25, no. 15: 3325. https://doi.org/10.3390/molecules25153325
APA StyleCham, B. T., Linh, N. T. T., Thao, D. T., Anh, N. T. H., Tam, N. T., Anh, B. K., Muscari, I., Adorisio, S., Sung, T. V., Thuy, T. T., & Delfino, D. V. (2020). Cell Growth Inhibition of Saponin XII from Dipsacus japonicus Miq. on Acute Myeloid Leukemia Cells. Molecules, 25(15), 3325. https://doi.org/10.3390/molecules25153325