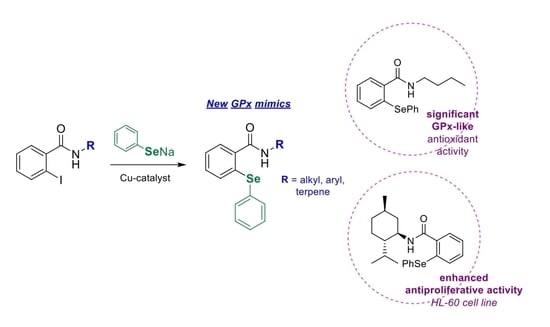
3.2.1. Synthesis of N-substituted o-iodobenzamides 7a–23a
2% NaOH (4.4 mL) was added to a solution of an amine (1.0 mmol) in DCM (2 mL). The mixture was cooled to 0 °C and o-iodobenzoic acid chloride (1.1 mmol) dissolved in DCM (3 mL) was added dropwise. The reaction mixture was stirred at room temperature for 20 h and the product was extracted with DCM. Combined organic layers were washed with saturated NaHCO3 and dried over magnesium sulfate. The solvent was removed under reduced pressure and the product was obtained as white solid.
((−)-N-(1R,2S,5R)-menthyl)-o-iodobenzamide18a Yield: 98%, mp 146–148 °C; [α = −38.93 (c = 5.73, CHCl3); 1H NMR (700 MHz, CDCl3) δ = 0.88 (d, J = 7.0 Hz, 3H, CH3), 0.92 (s, 3H, CH3), 0.93 (s, 3H, CH3), 0.98–1.03 (m, 1H), 1.11–1.18 (m, 2H), 1.51–1.57 (m, 2H), 1.69–1.75 (m, 2H), 2.08–2.12 (m, 1H), 2.17–2.20 (m, 1H), 3.95–4.00 (m, 1H), 5.41 (d, J = 9.1 Hz, 1H, NH), 7.06–7.09 (m, 1Har), 7.35–7.38 (m, 2Har), 7.84 (dd, J1 = 0.7, J2 = 7.7 Hz, 1Har); 13C NMR (100.6 MHz, CDCl3) δ = 16.20 (CH3), 21.20 (CH3), 22.15 (CH3), 23.78 (CH2), 26.91 (CH), 31.89 (CH), 34.52 (CH2), 42.86 (CH2), 48.13 (CH), 50.75 (CH), 92.32 (Car), 128.08 (CHar), 128.12 (CHar), 130.86 (CHar), 139.88 (CHar), 142.93 (Car), 168.61 (C=O); IR: 3230, 2951, 2916, 2867, 1636, 1584, 1540, 1462, 1430, 1385, 1367, 1341, 1325, 1307, 1261, 1161, 1147, 1116, 1107, 1059, 1043, 1014 cm−1; Elemental Anal. Calcd for C17H24INO (385.09): C, 53.00; H, 6.28; N, 3.64 Found: C, 53.18; H, 6.34; N, 3.76.
((−)-N-(1S,2R,3S,6R)-(2-caranyl))-o-iodobenzamide19a Yield: 90%; mp 145–147 °C; [α = −21.47 (c = 4.77, CHCl3); 1H NMR (700 MHz, CDCl3) 0.64–0.68 (m, 1H), 0.94–1.01 (m, 1H), 1.02 (d, J = 7.7 Hz, 3H, CH3), 1.05 (s, 3H, CH3), 1.11 (s, 3H, CH3), 1.23–1.28 (m, 1H), 1.55–1.60 (m, 2H), 1.71–1.74 (m, 1H), 1.77–1.82 (m, 1H), 3.54–3.58 (m, 1H), 5.72 (d, J = 8.4 Hz, 1H, NH), 7.09 (dt, J1 = 2.1, J2 = 8.4 Hz, 1Har), 7.38 (dt, J1 = 0.7, J2 = 7.7 Hz, 1Har), 7.43 (dd, J1 = 2.1, J2 = 7.7 Hz, 1Har), 7.87 (dd, J1 = 0.7, J2 = 7.7 Hz, 1Har); 13C NMR (100.6 MHz, CDCl3) δ = 15.55 (2 × CH3), 17.59 (C), 19.03 (CH2), 20.28 (CH), 28.71 (CH), 29.25 (CH3), 30.83 (CH2), 34.92 (CH), 50.41 (CH), 92.38 (Car), 128.14 (CHar), 128.35 (CHar), 130.90 (CHar), 139.96 (CHar), 142.81 (C), 168.50 (C=O); IR: 3249, 2915, 2862, 1656, 1630, 1585, 1546, 1459, 1430, 1375, 1330, 1257, 1115, 1015 cm−1; Elemental Anal. Calcd for C17H22INO (383.27): C, 53.27; H, 5.79; N, 3.65 Found: C, 53.05; H, 5.71; N, 3.53.
N-bornyl-o-iodobenzamide20a Yield: 93%; mp 122–123 °C (lit. [
14] mp 119–121 °C); [α
= +11.01 (c = 5.54, CHCl
3);
1H NMR (700 MHz, CDCl
3) 0.90 (s, 3H, CH
3), 0.91–0.99 (m, 1H), 0.96 (s, 3H, CH
3), 0.99 (s, 3H, CH
3), 1.17–1.21 (m, 1H), 1.41–1.46 (m, 1H), 1.56–1.61 (m, 1H), 1.70 (t,
J = 9.1 Hz, 1H), 1.76–1.81 (m, 1H), 2.42–2.47 (m, 1H), 4.41–4.45 (m, 1H), 5.78 (d,
J = 8.4 Hz, 1H, NH), 7.07–7.10 (m, 1H
ar), 7.35–7.40 (m, 2H
ar), 7.85 (dd,
J1 = 0.7,
J2 = 7.7 Hz, 1H
ar);
13C NMR (100.6 MHz, CDCl
3)
δ = 13.96 (CH
3), 18.72 (CH
3), 19.81 (CH
3), 28.20 (CH
2), 28.37 (CH
2), 37.44 (CH
2), 44.92 (CH), 48.38 (C), 49.70 (C), 54.64 (CH), 92.40 (C
ar), 128.20 (CH
ar), 128.42 (CH
ar), 130.96 (CH
ar), 139.83 (CH
ar), 142.84 (C
ar), 169.45 (C=O); IR: 3319, 2981, 2950, 2877, 1642, 1584, 1561, 1510, 1479, 1459, 1429, 1388, 1374, 1361, 1310, 1290, 1262, 1228, 1205, 1172, 1154, 1115, 1063, 1046, 1012 cm
−1; Elemental Anal. Calcd for C
17H
22INO (383.27): C, 53.27; H, 5.79; N, 3.65 Found: C, 53.11; H, 5.65; N, 3.47.
(−)-N-(1S,2R,5S)-myrtanyl-o-iodobenzamide21a Yield: 83%; mp 142–144 °C; [α = −8.23 (c = 5.33, CHCl3); 1H NMR (400 MHz, CDCl3) 0.96 (d, J = 9.6 Hz, 1H), 1.11 (s, 3H, CH3), 1.24 (s, 3H, CH3), 1.55–1.64 (m, 1H), 1.88–2.10 (m, 5H), 2.35–2.44 (m, 2H), 3.47–3.51 (m, 2H), 5.79 (bs, 1H, NH), 7.09–7.13 (m, 1Har), 7.37–7.42 (m, 2Har), 7.87 (dd, J1 = 0.8, J2 = 8.4 Hz, 1Har); 13C NMR (100.6 MHz, CDCl3) δ = 17.56 (CH2), 21.56 (CH3), 26.00 (CH2), 27.99 (CH3), 33.13 (CH2), 38.74 (C), 41.22 (CH), 41.36 (CH), 43.92 (CH), 45.75 (CH2), 92.41 (Car), 128.16 (CHar), 128.31 (CHar), 130.98 (CHar), 139.84 (CHar), 142.67 (Car), 169.33 (C=O); IR: 3239, 2935, 2903, 2889, 2859, 1636, 1584, 1542, 1462, 1430, 1382, 1364, 1316, 1292, 1260, 1218, 1156, 1113, 1054, 1014 cm−1; Elemental Anal. Calcd for C17H22INO (383.27): C, 53.27; H, 5.79; N, 3.65 Found: C, 53.55; H, 5.84; N, 3.74.
(−)-N-(1R,2R,3R,5S)-isopinocamphyl-o-iodobenzamide22a Yield: 78%; mp 130–132 °C; [α = −18.67 (c = 4.88, CHCl3); 1H NMR (400 MHz, CDCl3) 0.91 (d, J = 10.0 Hz, 1H), 1.12 (s, 3H, CH3), 1.26 (s, 3H, CH3), 1.28 (s, 3H, CH3), 1.73–1.78 (m, 1H), 1.88–1.91 (m, 1H), 1.93–1.97 (m, 1H), 2.01–2.05 (m, 1H), 2.44–2.50 (m, 1H), 2.73–2.80 (m, 1H), 4.48–4.56 (m, 1H), 5.67 (bs, 1H, NH), 7.09–7.14 (m, 1Har), 7.38–7.45 (m, 2Har), 7.88 (dd, J1 = 0.8, J2 = 8.0 Hz, 1Har); 13C NMR (100.6 MHz, CDCl3) δ = 20.98 (CH3), 23.39 (CH3), 28.00 (CH3), 35.31 (CH2), 36.91 (CH2), 38.49 (C), 41.60 (CH), 46.20 (CH), 47.85 (CH), 48.58 (CH), 92.47 (Car), 128.22 (CHar), 128.37 (CHar), 130.94 (CHar), 139.78 (CHar), 142.68 (Car), 168.78 (C=O); IR: 3242, 2980, 2969, 2900, 2867, 1632, 1584, 1556, 1534, 1458, 1428, 1384, 1372, 1348, 1336, 1319, 1301, 1259, 1227, 1160, 1056, 1016 cm−1; Elemental Anal. Calcd for C17H22INO (383.27): C, 53.27; H, 5.79; N, 3.65 Found: C, 53.49; H, 5.87; N, 3.71.
(+)-N-(1R,2R,3R,5S)-isopinocamphyl-o-iodobenzamide23a Yield: 79%; mp 142–144 °C; [α = +17.17 (c = 6.00, CHCl3) 1H NMR (400 MHz, CDCl3) 0.90 (d, J = 10.0 Hz, 1H), 1.11 (s, 3H, CH3), 1.25 (s, 3H, CH3), 1.27 (s, 3H, CH3), 1.72–1.77 (m, 1H), 1.88–1.90 (m, 1H), 1.94–1.96 (m, 1H), 2.02–2.03 (m, 1H), 2.43–2.49 (m, 1H), 2.72–2.79 (m, 1H), 4.49–4.55 (m, 1H), 5.65 (bs, 1H, NH), 7.09–7.13 (m, 1H, 1Har), 7.40–7.44 (m, 2H, 2Har), 7.87 (d, J = 7.2 Hz, 1H, 1Har); 3C NMR (100.6 MHz, CDCl3) δ = 20.99 (CH3), 23.40 (CH3), 28.00 (CH3), 35.33 (CH2), 36.94 (CH2), 38.50 (C), 41.60 (CH), 46.22 (CH), 47.83 (CH), 48.58 (CH), 92.49 (Car), 128.26 (CHar), 128.41 (CHar), 130.97 (CHar), 139.80 (CHar), 142.68 (Car), 168.80 (C=O); IR: 3305, 2962, 2922, 2891, 2863, 1632, 1582, 1526, 1450, 1428, 1378, 1347, 1334, 1313, 1294, 1274, 1257, 1229, 1220, 1163, 1015 cm−1; Elemental Anal. Calcd for C17H22INO (383.27): C, 53.27; H, 5.79; N, 3.65 Found: C, 53.59; H, 5.70; N, 3.78.
3.2.2. Synthesis of N-substituted phenylselenides 10–15
To a solution of a diphenyl diselenide (0.5 mmol) in dry toluene (5 mL), sodium borohydride (1.5 mmol) was added and stirred at room temperature. Next, DMSO was added dropwise until the solution discolored. Then, respectively, CuI (0.1 mmol), 1,10-phenanthroline (0.2 mmol) and an amide (1.0 mmol) were added. The mixture was stirred under reflux for 18 h. The solution was cooled to room temperature and brine (5 mL) was added. The product was extracted with chloroform (2 × 10 mL), and the combined organic layers were washed with water (2 × 10 mL), brine (2 × 10 mL) and dried over magnesium sulphate. The solvent was removed under reduced pressure and the obtained crude product was isolated by column chromatography (silica gel, DCM).
N-ethyl-2-(phenylselanyl)benzamide7b Yield: 38%; mp 89–91 °C (lit. [
20] mp 84–86 °C);
1H NMR (400 MHz, CDCl
3) δ = 1.27 (t,
J = 7.2 Hz, 3H, CH
3), 3.49–3.56 (m, 2H, N-CH
2), 6.08 (bs, 1H, NH), 7.11–7.13 (m, 1H
ar), 7.17–7.22 (m, 2H
ar), 7.35–7.41 (m, 3H
ar), 7.51–7.54 (m, 1H
ar), 7.63–7.65 (m, 2H
ar);
13C NMR (100.6 MHz, CDCl
3) δ = 14.81 (CH
3), 35.03 (CH
2), 125.80 (CH
ar), 127.36 (CH
ar), 128.48 (CH
ar), 129.59 (2 × CH
ar), 130.01 (C
ar), 130.90 (CH
ar), 131.34 (CH
ar), 134.79 (C
ar), 134.82 (C
ar), 135.95 (2 × CH
ar), 168.19 (C=O);
77Se NMR (76 MHz, CDCl
3) δ = 435.56 ppm; IR: 3267, 3069, 2971, 2927, 2869, 1621, 1585, 1553, 1462, 1448, 1437, 1377, 1359, 1311, 1286, 1261, 1167, 1145, 1121, 1093, 1063, 1033, 1018 cm
−1; Elemental Anal. Calcd for C
15H
15NOSe (305.04): C, 59.22; H, 4.97; N, 4.60 Found: C, 59.15; H, 4.89; N, 4.53.
N-propyl-2-(phenylselanyl)benzamide8b Yield: 41%; mp 74–76 °C (lit. [
21] mp 78–79 °C);
1H NMR (400 MHz, CDCl
3) δ = 1.01 (t,
J = 7.6 Hz, 3H, CH
3), 1.63–1.69 (m, 2H, CH
2), 3.19–3.46 (m, 2H, N-CH
2), 6.22 (bs, 1H, NH), 7.09–7.11 (m, 1H, 1H
ar), 7.16–7.20 (m, 2H, 2H
ar), 7.34–7.40 (m, 3H, 3H
ar), 7.51–7.54 (m, 1H, 1H
ar), 7.62–7.65 (m, 2H, 2H
ar);
13C NMR (100.6 MHz, CDCl
3) δ = 11.51 (CH
3), 22.88 (CH
2), 41.87 (CH
2), 125.76 (CH
ar), 127.39 (CH
ar), 128.51 (CH
ar), 129.59 (2 × CH
ar), 129.99 (C
ar), 130.89 (CH
ar), 131.21 (CH
ar), 134.79 (C
ar), 134.89 (C
ar), 136.03 (2 × CH
ar), 168.35 (C=O);
77Se NMR (76 MHz, CDCl
3) δ = 436.03 ppm; IR: 3277, 3054, 2960, 2922, 2870, 1618, 1585, 1549, 1458, 1436, 1380, 1359, 1313, 1288, 1259, 1145, 1100, 1066, 1033, 1017 cm
−1; Elemental Anal. Calcd for C
16H
17NOSe (319.05): C, 60.38; H, 5.38; N, 4.40 Found: C, 60.55; H, 5.42; N, 4.45.
N-butyl-2-(phenylselanyl)benzamide9b Yield: 60%; mp 121–125 °C; 1H NMR (700 MHz, CDCl3) δ = 0.96 (t, J = 7.0 Hz, 3H, CH3), 1.40–1.44 (m, 2H, CH2), 1.58–1.61 (m, 2H, CH2), 3.45–3.48 (m, 2H, N-CH2), 6.09 (bs, 1H, NH), 7.07–7.09 (m, 1Har), 7.16–7.19 (m, 2Har), 7.34–7.38 (m, 3Har), 7.49–7.50 (m, 1Har), 7.61–7.63 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 13.39 (CH3), 19.79 (CH2), 31.24 (CH2), 39.49 (CH2), 125.38 (CHar), 126.97 (CHar), 128.09 (CHar), 129.18 (2 × CHar), 129.59 (Car), 130.48 (CHar), 130.87 (CHar), 134.40 (Car), 134.45 (Car), 135.57 (2 × CHar), 167.87 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 435.10 ppm; IR: 3277, 3054, 2960, 2922, 2870, 1618, 1585, 1549, 1458, 1436, 1380, 1359, 1313, 1288, 1259, 1145, 1100, 1066, 1033, 1017 cm−1; Elemental Anal. Calcd for C17H19NOSe (333.06): C, 61.45; H, 5.76; N, 4.22 Found: C, 61.29; H, 5.69; N, 4.16.
N-hexyl-2-(phenylselanyl)benzamide11b Yield: 40%; mp 87–89 °C; 1H NMR (400 MHz, CDCl3) δ = 0.92(t, J = 7.2 Hz, 3H, CH3), 1.31–1.42 (m, 6H, 3 × CH2), 1.59–1.66 (m, 2H, CH2), 3.43–3.48 (m, 2H, N-CH2), 6.27 (bs, 1H, NH), 7.08–7.12 (m, 1Har), 7.16–7.19 (m, 2Har), 7.34–7.42 (m, 3Har), 7.52–7.54 (m, 1Har), 7.62–7.65 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 14.05 (CH3), 22.58 (CH2), 26.71 (CH2), 29.56 (CH2), 31.52 (CH2), 40.22 (CH2), 125.76 (CHar), 127.46 (CHar), 128.49 (CHar), 129.58 (2 × CHar), 130.05 (Car), 130.85 (CHar), 131.20 (CHar), 134.84 (Car), 134.85 (Car), 136.00 (2 × CHar), 168.33 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 435.14 ppm; IR: 3317, 2957, 2918, 2927, 2871, 2852, 1617, 1585, 1543, 1462, 1434, 1376, 1331, 1306, 1265, 1200, 1189, 1154, 1032, 1020 cm−1; Elemental Anal. Calcd for C19H23NOSe (361.09): C, 63.33; H, 6.43; N, 3.89 Found: C, 63.19; H, 6.34; N, 3.70.
N-(3-methylbutyl)-2-(phenylselanyl)benzamide10b Yield: 54%; mp 76–78 °C; 1H NMR (700 MHz, CDCl3) δ = 0.95 (d, J = 7.0 Hz, 6H, 2 × CH3), 1.49–1.52 (m, 2H, CH2), 1.67–1.72 (m, 1H, CH), 3.46–3.49 (m, 2H, N-CH2), 6.05 (bs, 1H, NH), 7.08–7.09 (m, 1H, 1Har), 7.16–7.19 (m, 2Har), 7.34–7.38 (m, 3Har), 7.48–7.50 (m, 1Har), 7.61–7.62 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 22.49 (CH3), 25.99 (CH), 38.43 (CH2), 38.49 (CH2), 125.83 (CHar), 127.38 (CHar), 128.47 (CHar), 129.59 (2 × CHar), 130.04 (Car), 130.88 (CHar), 131.39 (CHar), 134.68 (Car), 134.96 (Car), 135.90 (2 × CHar), 168.23 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 435.83 ppm; IR: 3315, 2955, 2927, 2870, 1624, 1585, 1564, 1564, 1536, 1462, 1434, 1384, 1364, 1342, 1304, 1284, 1268, 1255, 1226, 1158, 1023 cm−1; Elemental Anal. Calcd for C18H21NOSe (347.08): C, 62.43; H, 6.11; N, 4.04 Found: C, 62.61; H, 6.19; N, 4.13.
N-cyclohexyl-2-(phenylselanyl)benzamide12b Yield: 60%; mp 183–185 °C (lit. [
22] mp 179–181 °C);
1H NMR (400 MHz, CDCl
3) δ = 1.21–1.31 (m, 3H), 1.40–1.50 (m, 2H, CH
2), 1.65–1.69 (m, 1H), 1.74–1.80 (m, 2H, CH
2), 2.05–2.09 (m, 2H, CH
2), 3.97–4.07 (m, 1H, N-CH), 5.32 (bs, 1H, NH), 7.09–7.11 (m, 1H
ar), 7.17–7.23 (m, 2H
ar), 7.35–7.41 (m, 3H
ar), 7.50–7.54 (m, 1H
ar), 7.60–7.65 (m, 2H
ar);
13C NMR (100.6 MHz, CDCl
3) δ = 24.83 (2 × CH
2), 25.55 (CH
2), 33.07 (2 × CH
2), 48.87 (CH), 125.76 (CH
ar), 127.33 (CH
ar), 128.42 (CH
ar), 129.55 (2 × CH
ar), 130.01 (C
ar), 130.80 (CH
ar), 131.26 (CH
ar), 134.60 (C
ar), 135.04 (C
ar), 135.88 (2 × CH
ar), 167.38 (C=O);
77Se NMR (76 MHz, CDCl
3) δ = 434.17 ppm; IR: 3251, 3053, 2924, 2849, 1618, 1583, 1541, 1459, 1448, 1436, 1377, 1337, 1299, 1283, 1256, 1240, 1191, 1149, 1120, 1081, 1066, 1030, 1018 cm
−1; Elemental Anal. Calcd for C
19H
21NOSe (359.08): C, 63.68; H, 5.91; N, 3.91 Found: C, 63.52; H, 5.86; N, 3.83.
N-phenyl-2-(phenylselanyl)benzamide13b Yield: 90%; mp 139–140 °C (lit. [
23] mp 139–141 °C);
1H NMR (700 MHz, CDCl
3) 7.14–7.18 (m, 2H
ar), 7.22–7.26 (m, 2H
ar), 7.33–7.39 (m, 5H
ar), 7.59–7.61 (m, 4H
ar), 7.65–7.67 (m, 1H
ar), 7.87 (bs, 1H, NH);
13C NMR (75.5 MHz, CDCl
3)
δ = 120.18 (2 × CH
ar), 124.67 (CH
ar), 126.16 (CH
ar), 127.76 (CH
ar), 128.57 (CH
ar), 129.05 (2 × CH
ar), 129.65 (2 × CH
ar), 129.76 (C
ar), 131.33 (CH
ar), 131.86 (CH
ar), 134.86 (C
ar), 135.05 (C
ar), 135.79 (2 × CH
ar), 137.71 (C
ar), 166.33 (C=O);
77Se NMR (76 MHz, CDCl
3) δ = 434.60 ppm; IR: 3328, 3046, 1641, 1596, 1581, 1519, 1494, 1435, 1319, 1290, 1271, 1251, 1177, 1152, 1139, 1104, 1074, 1063, 1045, 1025 cm
−1; Elemental Anal. Calcd for C
19H
15NOSe (353.03): C, 64.78; H, 4.29; N, 3.98 Found: C, 64.92; H, 4.34; N, 4.09.
N-(p-chlorophenyl)-2-(phenylselanyl)benzamide14b Yield: 44%; mp 167–169 °C; 1H NMR (700 MHz, CDCl3) 7.20–7.21 (m, 1Har), 7.24–7.28 (m, 2Har), 7.32–7.38 (m, 5Har), 7.53–7.55 (m, 2Har), 7.58–7.59 (m, 2Har), 7.66 (dd, J1 = 1.4, J2 = 7.0 Hz, 1Har), 7.84 (bs, 1H, NH); 13C NMR (75.5 MHz, CDCl3) δ = 121.32 (2 × CHar), 126.32 (CHar), 127.83 (CHar), 128.61 (CHar), 129.08 (2 × CHar), 129.65 (2 × Car), 129.69 (2 × CHar), 131.52 (CHar), 132.11 (CHar), 134.68 (Car), 135.60 (2 × CHar), 136.24 (2 × Car), 166.21 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 433.36 ppm; IR: 3349, 3053, 1656, 1592, 1579, 1509, 1491, 1457, 1433, 1394, 1354, 1311, 1289, 1235, 1180, 1140, 1118, 1093, 1074, 1046, 1030, 1012, 1000 cm−1; Elemental Anal. Calcd for C19H14ClNOSe (386.99): C, 59.01; H, 3.65; N, 3.62 Found: C, 59.32; H, 3.55; N, 3.76.
N-(p-bromophenyl)-2-(phenylselanyl)benzamide15b Yield: 60%; mp 176–178 °C; 1H NMR (400 MHz, CDCl3) 7.22–7.24 (m, 1Har), 7.27–7.31 (m, 2Har), 7.35–7.41 (m, 3Har), 7.48–7.53 (m, 4Har), 7.60–7.62 (m, 2Har), 7.68–7.70 (m, 1Har), 7.86 (bs, 1H, NH); 13C NMR (75.5 MHz, CDCl3) 121.62 (2 × CHar), 126.37 (CHar), 127.86 (CHar), 128.58 (CHar), 129.68 (2 × CHar), 129.98 (2 × Car), 131.51 (CHar), 132.02 (2 × CHar), 132.22 (CHar), 134.53 (Car), 134.88 (Car), 135.51 (2 × CHar), 136.76 (Car), 166.18 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 433.40 ppm; IR: 3277, 1644, 1589, 1512, 1487, 1457, 1436, 1428, 1390, 1312, 1286, 1250, 1237, 1179, 1164, 1139, 1071, 1030, 1021, 1006 cm−1; Elemental Anal. Calcd for C19H14BrNOSe (430.94): C, 52.93; H, 3.27; N, 3.25 Found: C, 53.11; H, 3.35; N, 3.40.
N-(p-iodophenyl)-2-(phenylselanyl)benzamide16b Yield: 22%; mp 179–181 °C; 1H NMR (700 MHz, CDCl3) 7.18–7.20 (m, 1Har), 7.23–7.25 (m, 2Har), 7.33–7.35 (m, 2Har), 7.36–7.38 (m, 3Har), 7.57–7.59 (m, 2Har), 7.62–7.65 (m, 2Har), 7.94 (bs, 1H, NH); 13C NMR (75.5 MHz, CDCl3) 121.87 (2 × CHar), 126.35 (CHar), 127.85 (CHar), 128.60 (CHar), 129.68 (2 × CHar), 129.98 (2 × Car), 131.53 (CHar), 132.19 (CHar), 134.60 (Car), 134.53 (Car), 135.54 (2 × CHar), 137.47 (Car), 137.98 (2 × CHar), 166.18 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 433.36 ppm; IR: 3348, 3049, 2922, 2852, 1651, 1585, 1506, 1486, 1456, 1436, 1429, 1387, 1352, 1313, 1288, 1233, 1187, 1166, 1139, 1120, 1063, 1045, 1029, 1019, 1002 cm−1; Elemental Anal. Calcd for C19H14INOSe (478.93): C, 47.72; H, 2.95; N, 2.93 Found: C, 47.59; H, 3.01; N, 3.10.
N-(p-metoxyphenyl)-2-(phenylselanyl)benzamide17b Yield: 45%; mp 131–132 °C; 1H NMR (700 MHz, CDCl3) 3.83 (s, 3H, CH3), 6.71 (ddd, J1 = 0.7, J2 = 2.8 Hz, J2 = 8.4 Hz, 1Har), 7.05 (dd, J1 = 1.4, J2 = 8.4 Hz, 1Har), 7.18 (dd, J1 = 0.7, J2 = 7.7 Hz, 1Har), 7.23–7.28 (m, 3Har), 7.33–7.39 (m, 4Har), 7.60–7.61 (m, 2Har), 7.65 (dd, J1 = 1.4, J2 = 7.7 Hz, 1Har), 7.80 (bs, 1H, NH); 13C NMR (75.5 MHz, CDCl3) 55.37 (CH3), 105.74 (CHar), 110.66 (CHar), 112.22 (CHar), 126.22 (CHar), 127.77 (CHar), 128.54 (CHar), 129.64 (2 × CHar), 129.71 (CHar), 131.34 (CHar), 131.96 (CHar), 134.64 (Car), 135.18 (Car), 136.13 (2 × CHar), 138.91 (2 × Car), 160.22 (Car), 166.26 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 433.96 ppm; IR: 3300, 2922, 1640, 1597, 1581, 1564, 1521, 1489, 1464, 1453, 1427, 1306, 1287, 1273, 1253, 1201, 1172, 1156, 1140, 1038, 1022 cm−1; Elemental Anal. Calcd for C20H17NO2Se (383.04): C, 62.83; H, 4.48; N, 3.66 Found: C, 62.69; H, 4.39; N, 3.50.
(−)-N-(1R,2S,5R)-menthyl-2-(phenylselanyl)benzamide18b Yield: 27%, mp 153–155 °C; [α = −31.11 (c = 2.61, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 0.88 (d, J = 6.8 Hz, 3H, CH3), 0.93 (d, J = 6.4 Hz, 3H, CH3), 0.94 (d, J = 6.8 Hz, 3H, CH3), 1.16–1.20 (m, 2H), 1.51–1.59 (m, 3H), 1.71–1.81 (m, 2H), 2.01–2.09 (m, 1H), 2.12–2.18 (m, 1H), 3.95–4.04 (m, 1H), 5.78 (d, J = 8.8 Hz, 1H, NH), 7.10–7.12 (m, 1Har), 7.17–7.23 (m, 2Har), 7.34–7.40 (m, 3Har), 7.50–7.52 (m, 1Har), 7.62–7.64 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 16.26 (CH3), 21.25 (CH3), 22.17 (CH3), 23.85 (CH2), 26.99 (CH), 31.93 (CH), 34.56 (CH2), 43.02 (CH2), 48.25 (CH), 50.63 (CH), 125.79 (CHar), 127.26 (CHar), 128.41 (CHar), 129.57 (2 × CHar), 130.12 (Car), 130.76 (CHar), 131.33 (CHar), 134.51 (Car), 135.42 (Car), 135.86 (2 × CHar), 167.60 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 434.56 ppm; IR: 3364, 2960, 2936, 2863, 1637, 1582, 1522, 1458, 1435, 1338, 1305, 1255, 1157, 1031, 1020 cm−1; Elemental Anal. Calcd for C23H29NOSe (415.14): C, 66.66; H, 7.05; N, 3.38 Found: C, 66.78; H, 7.13; N, 3.23.
(−)-N-(1S,2R,3S,6R)-(2-caranyl)-2-(phenylselanyl)benzamide19b Yield: 53%; mp 115–116 °C; [α = −15.11 (c = 6.15, CHCl3); 1H NMR (400 MHz, CDCl3) 0.59–0.62 (m, 1H), 0.65–0.69 (m, 1H), 1.00 (d, J = 6.4 Hz, 3H, CH3), 1.05 (s, 3H, CH3), 1.13 (s, 3H, CH3), 1.24–1.32 (m, 1H), 1.55–1.62 (m, 1H), 1.72–1.88 (m, 3H), 3.59–3.65 (m, 1H), 6.15 (d, J = 8.8 Hz, 1H, NH), 7.10–7.13 (m, 1Har), 7.17–7.24 (m, 2Har), 7.35–7.42 (m, 3Har), 7.57–7.59 (m, 1Har), 7.64–7.67 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 15.55 (CH3), 17.58 (C), 18.88 (CH3), 19.09 (CH2), 20.25 (CH), 28.88 (CH), 29.30 (CH3), 30.80 (CH2), 35.32 (CH), 50.21 (CH), 125.77 (CHar), 127.38 (CHar), 128.43 (CHar), 129.58 (2 × CHar), 130.10 (Car), 130.79 (CHar), 131.32 (CHar), 134.72 (Car), 135.25 (Car), 135.94 (2 × CHar), 167.41 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 435.56 ppm; IR: 3233, 2920, 2863, 1623, 1582, 1540, 1477, 1459, 1437, 1375, 1331, 1284, 1258, 1243, 1161, 1114, 1086, 1055, 1021 cm−1; Elemental Anal. Calcd for C23H27NOSe (413.13): C, 66.98; H, 6.60; N, 3.40 Found: C, 67.12; H, 6.69; N, 3.51.
N-borynyl-2-(phenylselanyl)benzamide20b Yield: 46%; mp 118–120 °C; [α = −32.10 (c = 4.71, CHCl3); 1H NMR (400 MHz, CDCl3) 0.93 (s, 3H, CH3), 0.94 (s, 3H, CH3), 1.02 (s, 3H, CH3), 1.17–1.24 (m, 1H), 1.42–1.51 (m, 1H), 1.55–1.62 (m, 2H), 1.72–1.74 (m, 1H), 1.76–1.86 (m, 1H), 2.44–2.52 (m, 1H), 4.46–4.52 (m, 1H), 5.32 (d, J = 8.8 Hz, 1H, NH), 7.12–7.14 (m, 1Har), 7.19–7.26 (m, 2Har), 7.34–7.40 (m, 3Har), 7.56–7.58 (m, 1Har), 7.61–7.63 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 13.81 (CH3), 18.71 (CH3), 19.84 (CH3), 28.27 (CH2), 28.43 (CH2), 37.72 (CH2), 44.97 (CH), 48.26 (C), 49.72 (C), 54.48 (CH), 126.00 (CHar), 127.51 (CHar), 128.39 (CHar), 129.60 (2 × CHar), 130.09 (Car), 130.87 (CHar), 131.64 (CHar), 134.09 (Car), 135.57 (Car), 135.62 (2 × CHar), 168.36 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 432.53 ppm; IR: 3359, 2950, 2885, 1629, 1582, 1561, 1516, 1476, 1456, 1435, 1389, 1374, 1363, 1309, 1277, 1256, 1223, 1204, 1173, 1152, 1109, 1065, 1030, 1021 cm−1; Elemental Anal. Calcd for C23H27NOSe (413.13): C, 66.98; H, 6.60; N, 3.40 Found: C, 66.79; H, 6.54; N, 3.32.
(−)-N-(1S,2R,5S)-myrtanyl-2-(phenylselanyl)benzamide21b Yield: 55%; mp 115–117 °C; [α = −8.00 (c = 2.70, CHCl3); 1H NMR (400 MHz, CDCl3) 0.94 (d, J = 9.6 Hz, 1H), 1.11 (s, 3H, CH3), 1.23 (s, 3H, CH3), 1.53–1.63 (m, 2H), 1.87–2.06 (m, 4H), 2.31–2.43 (m, 2H), 3.47–3.51 (m, 2H), 6.13 (bs, 1H, NH), 7.10–7.13 (m, 1Har), 7.17–7.23 (m, 2Har), 7.35–7.42 (m, 3Har), 7.51–7.53 (m, 1Har), 7.62–7.64 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 19.94 (CH2), 23.25 (CH3), 26.02 (CH2), 27.99 (CH3), 33.24 (CH2), 38.75 (C), 41.39 (CH), 41.41 (CH), 43.91 (CH), 45.73 (CH2), 125.86 (CHar), 127.41 (CHar), 128.43 (CHar), 129.58 (2 × CHar), 130.06 (Car), 130.86 (CHar), 131.44 (CHar), 134.52 (Car), 135.16 (Car), 135.83 (2 × CHar), 168.27 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 434.53 ppm; IR: 3333, 2980, 2905, 1631, 1584, 1560, 1530, 1462, 1432, 1383, 1364, 1314, 1284, 1254, 1219, 1154, 1058, 1032, 1019, 1001 cm−1; Elemental Anal. Calcd for C23H27NOSe (413.13): C, 66.98; H, 6.60; N, 3.40 Found: C, 67.12; H, 6.69; N, 3.50.
(−)-N-(1R,2R,3R,5S)-isopinocamphyl-2-(phenylselanyl)benzamide22b Yield: 53%; mp 117–119 °C; [α = −14.75 (c = 4.73, CHCl3); 1H NMR (400 MHz, CDCl3) 0.88 (d, J = 10.0 Hz, 1H), 1.11 (s, 3H, CH3), 1.22 (d, J = 7.2 Hz, 3H, CH3), 1.27 (s, 3H, CH3), 1.63–1.69 (m, 1H), 1.87–1.89 (m, 2H), 2.00–2.03 (m, 1H), 2.43–2.49 (m, 1H), 2.72–2.79 (m, 1H), 4.48–4.56 (m, 1H), 6.01 (d, J = 7.2 Hz, 1H, NH), 7.12–7.14 (m, 1Har), 7.18–7.25 (m, 2Har), 7.35–7.40 (m, 3Har), 7.54–7.56 (m, 1Har), 7.61–7.64 (m, 2Har); 13C NMR (100.6 MHz, CDCl3) δ = 20.88 (CH3), 23.38 (CH3), 28.01 (CH3), 35.34 (CH2), 37.21 (CH2), 38.45 (C), 41.62 (CH), 46.49 (CH), 47.85 (CH), 48.53 (CH), 125.97 (CHar), 127.51 (CHar), 128.39 (CHar), 129.61 (2 × CHar), 130.08 (Car), 130.84 (CHar), 131.55 (CHar), 134.10 (Car), 135.44 (Car), 135.63 (2 × CHar), 167.72 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 431.80 ppm; IR: 3303, 2953, 2903, 2868, 1617, 1582, 1559, 1528, 1475, 1461, 1434, 1375, 1337, 1318, 1300, 1286, 1255, 1226, 1160, 1062, 1031, 1021 cm−1; Elemental Anal. Calcd for C23H27NOSe (413.13): C, 66.98; H, 6.60; N, 3.40 Found: C, 66.78; H, 6.52; N, 3.34.
(+)-N-(1R,2R,3R,5S)-isopinocamphyl-2-(phenylselanyl)benzamide23b Yield: 54%; mp 128–130 °C; [α = +14.35 (c = 4.43, CHCl3); 1H NMR (400 MHz, CDCl3) 0.89 (d, J = 10.0 Hz, 1H), 1.11 (s, 3H, CH3), 1.21 (d, J = 7.2 Hz, 3H, CH3), 1.26 (s, 3H, CH3), 1.63–1.69 (m, 1H), 1.86–1.93 (m, 2H), 1.99–2.06 (m, 1H), 2.42–2.48 (m, 1H), 2.71–2.78 (m, 1H), 4.48–4.56 (m, 1H), 6.09 (d, J = 7.2 Hz, 1H, NH), 7.09–7.15 (m, 1Har), 7.16–7.23 (m, 2Har), 7.34–7.41 (m 3Har), 7.53–7.57 (m, 1Har), 7.59–7.64 (m, 2Har); 13C NMR (75.5 MHz, CDCl3) δ = 20.88 (CH3), 23.38 (CH3), 28.02 (CH3), 35.25 (CH2), 37.08 (CH2), 38.45 (C), 41.59 (CH), 46.21 (CH), 47.81 (CH), 48.48 (CH), 125.82 (CHar), 127.54 (CHar), 128.40 (CHar), 129.58 (2 × CHar), 130.08 (Car), 130.77 (CHar), 131.27 (CHar), 134.33 (Car), 135.19 (Car), 135.73 (2 × CHar), 167.74 (C=O); 77Se NMR (76 MHz, CDCl3) δ = 431.86 ppm; IR: 3311, 3055, 2958, 2908, 2869, 1623, 1584, 1563, 1531, 1476, 1454, 1434, 1376, 1351, 1335, 1311, 1298, 1285, 1258, 1226, 1163, 1092, 1064, 1031, 1021, 1000 cm−1; Elemental Anal. Calcd for C23H27NOSe (413.13): C, 66.98; H, 6.60; N, 3.40 Found: C, 66.68; H, 6.70; N, 3.51.







 18b
18b 33
33 19b
19b 34
34