Amburana cearensis: Pharmacological and Neuroprotective Effects of Its Compounds
Abstract
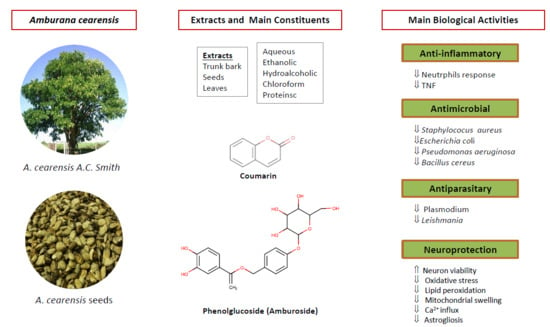
:1. Introduction
2. Botany
3. Toxicology
4. Chemical Constituents
5. Biological Activities
6. Neuroprotective Activities of A. cearensis Compounds
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marinho, M.G.V.; Silva, C.C.; Andrade, L.H.C. Levantamento etnobotânico de plantas medicinais em área de caatinga no município de São José de Espinharas, Paraíba, Brasil. Rev. Bras. Plantas Med. 2011, 13, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.R.G.S.; Guimarães, A.G.; Quintans, J.D.S.S.; Santos, M.R.V.; Lima, J.T.; Nunes, X.P.; Quintans-Júnior, L.J. Amburana cearensis–uma revisão química e farmacológica. Sci. Plena 2010, 6, 1–8. [Google Scholar]
- Canuto, K.M.; Silveira, E.R.; Bezerra, A.M.E. Estudo fitoquímico de espécimens cultivados de cumaru (Amburana cearensis A.C. Smith). Quim. Nova 2010, 33, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Melo, C.d.A.; Souza, P.O.; Damasceno, E. Atividade farmacológica da planta Amburana cearensis (imburana) frente a estudo etnofarmacológico em Monte Azul-Mg. Rev. Bras. Pesqui. em Ciências da Saúde 2014, 1, 31–34. [Google Scholar]
- Loureiro, M.B. Aspectos morfoanatômicos e fisiológicos de sementes e plântulas de Amburana cearensis (Fr. All.) A.C. Smith (Leguminosae-Papilionoideae). Rev. Árvore 2013, 37, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Zambrana, N.Y.P.; Zambrana, N.Y.P.; Bussmann, R.W. La Etnobotánica de los Chácobo en el siglo XXI. Ethnobot. Res. Appl. 2018, 16, 1–149. [Google Scholar] [CrossRef] [Green Version]
- Sá, M.B.; Ralph, M.T.; Nascimento, D.C.O.; Ramos, C.S.; Barbosa, I.M.S.; Sá, F.B.; Lima-Filho, J.V. Phytochemistry and preliminary assessment of the antibacterial activity of chloroform extract of Amburana cearensis (Allemão) A.C. Sm. against klebsiella pneumoniae carbapenemase-producing strains. Evid.-Based Complement. Altern. Med. 2014, 2014, 7. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.K.A.M.; Matos, M.E.; Matos, F.J.A.; Ribeiro, R.A.; Ferreira, F.V.; Viana, G.S.B. Antinociceptive and antiedematogenic effects of the hydroalcoholic extract and coumarin from Torresea cearensis Fr. All. Phytomedicine 1997, 4, 221–227. [Google Scholar] [CrossRef]
- Marinho, M.D.G.V.; De Brito, A.G.; Carvalho, K.D.A.; Bezerra-Santos, C.R.; Andrade, L.D.H.C.; Barbosa-Filho, J.M.; Piuvezam, M.R. Amburana cearensis e cumarina imunomodulam os níveis de anticorpos antígeno-especffico em camundongos BALB/c sensibilizados com ovalbumina. Acta Farm. Bonaer. 2004, 23, 47–52. [Google Scholar]
- Pereira, E.P.L.; Braga-De-Souza, S.; Santos, C.C.; Santos, L.O.; Cerqueira, M.D.; Ribeiro, P.R.; Fernandez, L.G.; Silva, V.D.A.; Costa, S.L. Amburana cearensis seed extracts protect PC-12 cells against toxicity induced by glutamate. Brazilian, J. Pharmacogn. 2017, 27, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Lima Pereira, É.P.; Santos Souza, C.; Amparo, J.; Short Ferreira, R.; Nuñez-Figueredo, Y.; Gonzaga Fernandez, L.; Ribeiro, P.R.; Braga-de-Souza, S.; Amaral da Silva, V.D.; Lima Costa, S. Amburana cearensis seed extract protects brain mitochondria from oxidative stress and cerebellar cells from excitotoxicity induced by glutamate. J. Ethnopharmacol. 2017, 209, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.K.A.M.; Nobre, H.V.; Cunha, G.M.A.; Moraes, M.O.; Pessoa, C.; Oliveira, R.A.; Silveira, E.R.; Canuto, K.M.; Viana, G.S.B. Amburoside A, a glucoside from Amburana cearensis, protects mesencephalic cells against 6-hydroxydopamine-induced neurotoxicity. Neurosci. Lett. 2005, 388, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Canuto, K.M.; Silveira, E.R. Constituíntes químicos da casca do caule de Amburana cearensis A.C. SMITH. Quim. Nova 2006, 29, 1241–1243. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.R.; Cavalcante, R.R.L.; Martins, M.C.C.; Parente, D.M.; Cavalcante, A.A.M.C. Avaliação da atividade antiedematogênica, antimicrobiana e mutagênica das sementes de Amburana cearensis (A.C. Smith) (Imburana-de-cheiro). Rev. Bras. Plantas Med. 2013, 15, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.d.C.L.; Ferreira, R.A. Aspectos morfológicos da semente e do desenvolvimento da planta jovem de Amburana cearensis (Arr. Cam.) A.C. Smith-Cumaru-Leguminosae Papilionoideae. Rev. Bras. Sementes 2003, 25, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Ramos, K.M.O.; Felfili, J.M.; Fagg, C.W.; Sousa-Silva, J.C.; Franco, A.C. Desenvolvimento inicial e repartição de biomassa de Amburana cearensis (Allemao) A.C. Smith, em diferentes condições de sombreamento. Acta Bot. Brasilica 2004, 18, 351–358. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho Nilo Bitu, V.; De Carvalho Nilo Bitu, V.; Matias, E.F.F.; De Lima, W.P.; Da Costa Portelo, A.; Coutinho, H.D.M.; De Menezes, I.R.A. Ethnopharmacological study of plants sold for therapeutic purposes in public markets in Northeast Brazil. J. Ethnopharmacol. 2015, 172, 265–272. [Google Scholar] [CrossRef]
- Maia de Morais, S.; Pereira Dantas, J.D.; Raquel Araújo da Silva, A.; Farias Magalhães, E. Plantas medicinais usadas pelos índios Tapebas do Ceará. Rev. Bras. Farmacogn. Brazilian, J. Pharmacogn. 2005, 15, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.K.A.; Sampaio, I.L.; Santana, G.S.M.; Bezerra, F.A.F.; Moraes, M.O.; Moraes, M.E.A. Clinical toxicology study of a herbal medicine formulation of Torresea cearensis in healthy volunteers. Rev. Bras. Plantas Med. 2007, 9, 55–60. [Google Scholar]
- Leal, L.K.A.M.; Oliveira, F.G.; Fontenele, J.B.; Ferreira, M.A.D.; Viana, G.S.B. Toxicological study of the hydroalcoholic extract from Amburana cearensis in rats. Pharm. Biol. 2003, 41, 308–314. [Google Scholar] [CrossRef]
- Costa-Lotufo, L.V.; Jimenez, P.C.; Wilke, D.V.; Leal, L.K.A.M.; Cunha, G.M.A.; Silveira, E.R.; Canuto, K.M.; Viana, G.S.B.; Moraes, M.E.A.; De Moraes, M.O.; et al. Antiproliferative Effects of Several Compounds Isolated from Amburana cearensis A.C. Smith. Zeitschrift fur Naturforsch. C 2003, 58, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Bravo B., J.A.; Sauvain, M.; Gimenez, A.; Muñoz, V.; Callapa, J.; Le Men-Olivier, L.; Massiot, G.; Lavaud, C. Bioactive phenolic glycosides from Amburana cearensis. Phytochemistry 1999, 50, 71–74. [Google Scholar]
- Negri, G.; Oliveira, A.F.M.; Salatino, M.L.F.; Salatino, A. Chemistry of the stem bark of Amburana cearensis (Allemão) (A.C.SM.). Rev. Bras. Plantas Med. 2004, 6, 1–4. [Google Scholar]
- Tanaka, A.S.; Sampaio, M.U.; Mentele, R.; Auerswald, E.A.; Sampaio, C.A.M. Sequence of a new bowman-birk inhibitor from Torresea acreana seeds and comparison with Torresea cearensis trypsin inhibitor (TcTI2). J. Protein Chem. 1996, 15, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.S.; Sampaio, M.U.; Oliva, M.L.V.; Sampaio, C.A.M.; Marangoni, S.; de Oliveira, B.; Novelle, J.C.; Fink, E. Purification and Primary Structure Determination of a Bowman-BirkTrypsin Inhibitor from Torresea cearensis Seeds. Biol. Chem. 1997, 378, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.F.; Tashima, A.K.; Pereira, R.M.S.; Mohamed, R.S.; Cabral, F.A. Coumarin solubility and extraction from emburana (Torresea cearensis) seeds with supercritical carbon dioxide. J. Supercrit. Fluids 2008, 43, 375–382. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as antioxidants. Mini Rev. Med. Chem. 2006, 6, 365–374. [Google Scholar] [CrossRef]
- Fylaktakidou, K.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolaides, D. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/Antioxidant Activities. Curr. Pharm. Des. 2005, 10, 3813–3833. [Google Scholar] [CrossRef]
- Gagliotti Vigil de Mello, S.V.; Frode, T.S. In Vitro and In Vivo Experimental Model-based Approaches for Investigating Anti-inflammatory Properties of Coumarins. Curr. Med. Chem. 2018, 25, 1446–1476. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.T.A.; Moura, G.M.M.; da Cruz, J.I.O.; Lima, R.V.C.; dos Santos, E.A.; Andrade, J.C.; Alencar, M.V.O.B.; Landim, V.P.A.; Coutinho, H.D.M.; Uchoa, A.F. Serine protease inhibition and modulatory-antibiotic activity of the proteic extract and fractions from Amburana cearensis. Food Chem. Toxicol. 2020, 135, 110946. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.F.; Cavalheiro, M.G.; Viana, M.P.; Queiroz, V.A.; Rocha-Bezerra, L.C.B.; Vasconcelos, I.M.; Morais, S.M.; Carvalho, A.F.U. Water extracts of Brazilian leguminous seeds as rich sources of larvicidal compounds against Aedes aegypti L. An. Acad. Bras. Cienc. 2010, 82, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberino, R.S.; Barros, V.R.P.; Menezes, V.G.; Santos, L.P.; Araújo, V.R.; Queiroz, M.A.A.; Almeida, J.R.G.S.; Palheta, R.C.; Matos, M.H.T. Amburana cearensis leaf extract maintains survival and promotes in vitro development of ovine secondary follicles. Zygote 2015, 24, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, B.B.; Macedo, T.J.S.; Santos, J.M.S.; Barberino, R.S.; Menezes, V.G.; Müller, M.C.; Almeida, J.R.G.S.; Figueiredo, J.R.; Matos, M.H.T. Supplemented base medium containing Amburana cearensis associated with FSH improves in vitro development of isolated goat preantral follicles. Theriogenology 2016, 86, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.; Fonseca, F.; Pereira, F.; Canuto, K.; Felipe, C.; Fontenele, J.; Pitombeira, M.; Silveira, E.; Viana, G. Protective Effects of Amburoside A, a Phenol Glucoside from Amburana cearensis, against CCl 4-Induced Hepatotoxicity in Rats. Planta Med. 2008, 74, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.K.A.M.; Ferreira, A.A.G.; Bezerra, G.A.; Matos, F.J.A.; Viana, G.S.B. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: A comparative study. J. Ethnopharmacol. 2000, 70, 151–159. [Google Scholar] [CrossRef]
- Leal, L.K.A.M.; Nechio, M.; Silveira, E.R.; Canuto, K.M.; Fontenele, J.B.; Ribeiro, R.A.; Viana, G.S.B. Anti-inflammatory and smooth muscle relaxant activities of the hydroalcoholic extract and chemical constituents from Amburana cearensis A.C. Smith. Phyther. Res. 2003, 17, 335–340. [Google Scholar] [CrossRef]
- de Araújo Lopes, A.; Magalhães, T.R.; de Andrade Uchôa, D.E.; Silveira, E.R.; Azzolini, A.E.C.S.; Kabeya, L.M.; Lucisano-Valim, Y.M.; Vasconcelos, S.M.M.; de Barros Viana, G.S.; Leal, L.K.A.M. Afrormosin, an Isoflavonoid from Amburana cearensis A. C.; Smith, Modulates the Inflammatory Response of Stimulated Human Neutrophils. Basic Clin. Pharmacol. Toxicol. 2013, 113, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Kritsilis, M.; Rizou, S.V.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, cellular senescence and neurodegenerative disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloemd, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray Dorsey, E.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; De Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics-2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccocioppo, F.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Simeone, P.; Lanuti, P.; Pieragostino, D.; Del Boccio, P.; Marchisio, M.; Miscia, S. Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen. Res. 2020, 15, 850–856. [Google Scholar]
- Paris, I.; Cardenas, S.; Lozano, J.; Perez-Pastene, C.; Graumann, R.; Riveros, A.; Caviedes, P.; Segura-Aguilar, J. Aminochrome as a preclinical experimental model to study degeneration of dopaminergic neurons in Parkinson’s disease. Neurotox. Res. 2007, 12, 125–134. [Google Scholar] [CrossRef]
- Paris, I.; Lozano, J.; Perez-Pastene, C.; Muñoz, P.; Segura-Aguilar, J. Molecular and neurochemical mechanisms in PD pathogenesis. Neurotox. Res. 2009, 16, 271–279. [Google Scholar] [CrossRef]
- Dickson, D.W. Neuropathology of Parkinson disease. Park. Relat. Disord. 2018, 46, S30–S33. [Google Scholar] [CrossRef]
- Muñoz, P.; Cardenas, S.; Huenchuguala, S.; Bricen, A.; Couve, E.; Paris, I.; Segura-aguilar, J. DT-Diaphorase Prevents Aminochrome-Induced Alpha-Synuclein Oligomer Formation and Neurotoxicity. Toxicol. Sci. 2015, 145, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, P.S.; Segura-Aguilar, J. DT-diaphorase Protects Against Autophagy Induced by Aminochrome-Dependent Alpha-Synuclein Oligomers. Neurotox. Res. 2017, 32, 362–367. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Jakes, R.; Goedert, M. alpha-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Hashimoto, M.; Rockenstein, E.; Crews, L.; Masliah, E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol. Med. 2003, 4, 21–36. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R. Alzheimer’s disease: Glutamate depletion in the hippocampal perforant pathway zone. Ann. Neurol. 1987, 22, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, 1–22. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Salmon, D.P. Alzheimer’s disease: Past, present, and future. J. Int. Neuropsychol. Soc. 2017, 23, 818–831. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kao, S.-Y.; Lee, F.J.S.; Song, W.; Jin, L.-W.; Yankner, B.A. Dopamine-dependent neurotoxicity of α -synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002, 8, 600–606. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Michallon, C.H.U.; Pa, B.; Cedex, G. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’ s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative Damage Is the Earliest Event in Alzheimer Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef] [Green Version]
- Schapira, A.H.V.; Olanow, C.W.; Greenamyre, J.T.; Bezard, E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: Future therapeutic perspectives. Lancet 2014, 384, 545–555. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Reeve, A.K.; Ludtmann, M.H.R.; Angelova, P.R.; Simcox, E.M.; Horrocks, M.H.; Klenerman, D.; Gandhi, S.; Turnbull, D.M.; Abramov, A.Y. Aggregated α-synuclein and complex I deficiency: Exploration of their relationship in differentiated neurons. Cell Death Dis. 2015, 6, e1820. [Google Scholar] [CrossRef] [PubMed]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-agid, P.F.; Jenner, I.P.; Marsden, C.D.; Sian, J.; Dt, D.; et al. Alterations in Glutathione Levels in Padanson’s Disease and Other Neurodegenerative Disorders Afecting Basal Ganglia. Ann. Neurol. 1994, 36, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Dexter, D.T.; Sian, J.; Schapira, A.H.V.; Marsden, C.D. Oxidative Stress as a Cause of Nigral Cell Death in Parkmson’s Disease and Incidental Lewy Body Disease. Ann. Neurol. 1992, 32, S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; De Leon, M.; Murray, J.; Lez, E.; Lu, J.; Javier, E.; McHugh, P.; Swerdlow, R.H. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 483–490. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Xu, D.; Chen, H.; Mak, S.; Hu, S.; Tsim, K.W.K.; Hu, Y.; Sun, Y.; Zhang, G.; Wang, Y.; Zhang, Z.; et al. Neuroprotection against glutamate-induced excitotoxicity and induction of neurite outgrowth by T-006, a novel multifunctional derivative of tetramethylpyrazine in neuronal cell models. Neurochem. Int. 2016, 99, 194–205. [Google Scholar] [CrossRef]
- Pallo, S.P.; Dimaio, J.; Cook, A.; Nilsson, B.; Johnson, G.V.W. Mechanisms of tau and Aβ-induced excitotoxicity. Brain Res. 2016, 1634, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Butt, A.M.; Vanzulli, I.; Papanikolaou, M.; De La Rocha, I.C.; Hawkins, V.E. Metabotropic Glutamate Receptors Protect Oligodendrocytes from Acute Ischemia in the Mouse Optic Nerve. Neurochem. Res. 2017, 42, 2468–2478. [Google Scholar] [CrossRef] [Green Version]
- Berliocchi, L.; Bano, D.; Nicotera, P. Ca2+ signals and death programmes in neurons. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005, 360, 2255–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azim, K.; Angonin, D.; Marcy, G.; Pieropan, F.; Rivera, A.; Donega, V.; Cantù, C.; Williams, G.; Berninger, B.; Butt, A.M.; et al. Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol. 2017, 15, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda, C.B.; Llorente-Folch, I.; Traba, J.; Amigo, I.; Gonzalez-Sanchez, P.; Contreras, L.; Juaristi, I.; Martinez-Valero, P.; Pardo, B.; del Arco, A.; et al. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Chassain, C.; Bielicki, G.; Donnat, J.-P.; Renou, J.-P.; Eschalier, A.; Durif, F. Cerebral glutamate metabolism in Parkinson’s disease: An in vivo dynamic (13)C NMS study in the rat. Exp. Neurol. 2005, 191, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis—A new target in stroke? Neurosci. Lett. 2019, 689, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Postolache, T.T.; Wadhawan, A.; Can, A.; Lowry, C.A.; Woodbury, M.; Makkar, H.; Hoisington, A.J.; Scott, A.J.; Potocki, E.; Benros, M.E.; et al. Inflammation in traumatic brain injury. J. Alzheimer’s Dis. 2020, 74, 1–28. [Google Scholar] [CrossRef]
- Kearney, H.; Cryan, J.; Beausang, A.; Looby, S.; Brett, F.M. Reactive gliosis mimicking tumor recurrence-A case series documenting MRI abnormalities and neuropathological correlates. Clin. Neuropathol. 2018, 37, 97–104. [Google Scholar] [CrossRef]
- de Monasterio-Schrader, P.; Patzig, J.; Möbius, W.; Barrette, B.; Wagner, T.L.; Kusch, K.; Edgar, J.M.; Brophy, P.J.; Werner, H.B. Uncoupling of neuroinflammation from axonal degeneration in mice lacking the myelin protein tetraspanin-2. Glia 2013, 61, 1832–1847. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Y.; Cooper, C.; Liu, B.; Wilson, B.; Hong, J.S. Microglia enhance β-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J. Neurochem. 2002, 83, 973–983. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation Induces Neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [Green Version]
- Sommer, A.; Winner, B.; Prots, I. The Trojan horse-Neuroinflammatory impact of T cells in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blasko, I.; Apochal, A.; Boeck, G.; Hartmann, T.; Grubeck-Loebenstein, B.; Ransmayr, G. Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiol. Dis. 2001, 8, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Bassani, T.B.; Vital, M.A.B.F.; Rauh, L.K. Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arq. Neuropsiquiatr. 2015, 73, 616–623. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Song, G.J.; Suk, K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.L.; Silva, V.D.A.; dos Santos Souza, C.; Santos, C.C.; Paris, I.; Muñoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Souza, C.; Grangeiro, M.S.; Lima Pereira, E.P.; dos Santos, C.C.; da Silva, A.B.; Sampaio, G.P.; Ribeiro Figueiredo, D.D.; David, J.M.; David, J.P.; da Silva, V.D.A.; et al. Agathisflavone, a flavonoid derived from Poincianella pyramidalis (Tul.), enhances neuronal population and protects against glutamate excitotoxicity. Neurotoxicology 2018, 65, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, M.T.S.; Macedo, F.V.V.; Van De Meent, M.; Rhee, I.K.; Verpoorte, R. Seleção de plantas com atividade anticolinasterase para tratamento da doença de Alzheimer. Quim. Nova 2003, 26, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Knight, R.; Khondoker, M.; Magill, N.; Stewart, R.; Landau, S. A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement. Geriatr. Cogn. Disord. 2018, 45, 131–151. [Google Scholar] [CrossRef] [Green Version]
- Kamkwalala, A.R.; Newhouse, P.A. Beyond Acetylcholinesterase Inhibitors: Novel Cholinergic Treatments for Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 377–392. [Google Scholar] [CrossRef]
- Salari, S.; Bagheri, M. In vivo, in vitro and pharmacologic models of Parkinson’s disease. Physiol. Res. 2019, 68, 17–24. [Google Scholar] [CrossRef]
- Glinka, Y.; Gassen, M.; Youdim, M.B.H. Mechanism of 6-hydroxydopamine neurotoxicity. J. Neural Transm. Suppl. 1997, 50, 55–66. [Google Scholar]
- Hernandez-Baltazar, D.; Zavala-Flores, L.M.; Villanueva-Olivo, A. El modelo de 6-hidroxidopamina y la fisiopatología parkinsoniana: Nuevos hallazgos en un viejo modelo. Neurologia 2017, 32, 533–539. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Sciacca, S.; Lynch, J.; Davagnanam, I.; Barker, R. Midbrain, pons, and medulla: Anatomy and syndromes. Radiographics 2019, 39, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Wang, S.Y.; Wang, X.D.; Jiang, H.Q.; Yang, Y.Q.; Wang, Y.; Cheng, J.L.; Zhang, C.T.; Liang, W.W.; Feng, H.L. Fisetin Exerts Antioxidant and Neuroprotective Effects in Multiple Mutant hSOD1 Models of Amyotrophic Lateral Sclerosis by Activating ERK. Neuroscience 2018, 379, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Paracatu, L.C.; Zeraik, M.L.; de Carvalho Bertozo, L.; de Andrade Bartolomeu, A.; da Silva Filho, L.C.; da Fonseca, L.M.; Ximenes, V.F. Synthesis, Antioxidant and Anti-inflammatory Properties of an Apocynin-Derived Dihydrocoumarin. Med. Chem. (Los. Angeles) 2016, 13, 93–100. [Google Scholar]
- de Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; de Paula da Silva, C.H.T.; Rosa, J.M.C.; Cruz, R.A.S.; da Silva Hage-Melim, L.I. Antioxidant Effect of Flavonoids Present in Euterpe oleracea Martius and Neurodegenerative Diseases: A Literature Review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 75–99. [Google Scholar] [CrossRef]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.L.; Yang, H.; Wang, Y.H.; Du, G.H. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int. Immunopharmacol. 2018, 56, 29–35. [Google Scholar] [CrossRef]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorganic Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic compounds characteristic of the mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Beg, T.; Jyoti, S.; Naz, F.; Rahul; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord.-Drug Targets 2018, 17, 421–429. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Park, H.Y.; Badshah, H.; Rehman, S.U.; Kim, M.O. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol. Neurobiol. 2017, 54, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.S.; Souza, C.S.; Chicaybam, L.; Bonamino, M.H.; Bahia, M.; Costa, S.L.; Borges, H.L.; Rehen, S.K. Agathisflavone enhances retinoic acid-induced neurogenesis and its receptors α and β in pluripotent stem cells. Stem Cells Dev. 2011, 20, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lu, C.W.; Lin, T.Y.; Huang, S.K.; Wang, S.J. Baicalein, a Constituent of Scutellaria baicalensis, Reduces Glutamate Release and Protects Neuronal Cell Against Kainic Acid-Induced Excitotoxicity in Rats. Am. J. Chin. Med. 2016, 44, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Lin, T.Y.; Lu, C.W.; Huang, S.K.; Wang, Y.C.; Chou, S.S.P.; Wang, S.J. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology 2015, 50, 157–169. [Google Scholar] [CrossRef]

| Author (Year) | Plant Part | Extract | Isolated Compounds | Activity |
|---|---|---|---|---|
| Leal et al., 1997 | Bark | Hydroalcoholic extract | Coumarin; isokaempferol, flavonoids (fraction). | Hydroalcoholic extract and coumarin: anti-nociceptive; anti-inflammatory |
| Bravo B. et al., 1999 | Bark | Dichloromethane extract | Coumarin, Amburoside A, Amburoside B. | Extract: anti-plasmodium Coumarin: leishmanicidal, bactericidal and antimalarial Amburoside A: antimalarial |
| Leal et al., 2000 | Bark | Hydroalcoholic extract | - | Antinociceptive, anti-inflammatory and bronchodilator |
| Costa-Lotufo, 2003 | Bark | Methanolic extract | kaempferol, isokaempferide, amburoside A and protocatechuic acid | Isolated compounds: antiproliferative and antioxidant |
| Leal et al., 2003a | Bark | Hydroalcoholic extract | Coumarin; 3,4,5-trihydroxi-benzoic acid; isokaempferol; fisetin and a biflavonoid | Extract, coumarin and flavonoid fraction: anti-inflammatory; (bronchodilator). |
| Marinho et al., 2004 | Bark | Hydroalcoholic extract | Coumarin | Immunomodulation of specific antibodies; vascular permeability reduction. |
| Leal et al., 2005 | Bark | Ethanolic extract | Amburoside A | Amburoside A: neuroprotective |
| Leal et al., 2008 | Bark | Ethanolic extract | Amburoside A | Hepatoprotective and anti-inflammatory |
| Farias et al., 2010 | Seeds | Aqueous extract | - | Toxicity against Aedes aegypti larvae |
| Lima et al., 2013 | Seeds | Aqueous extract | - | Anti-edematous |
| Lopes et al., 2013 | Bark | Ethanolic extract | Afrormosin | Afrormosin: inhibition of neutrophil responses |
| Sá et al., 2014 | Bark | Chloroform extract | - | Extract: bacteriostatic |
| Barberino et al., 2015 | Leaves | Ethanolic extract | - | Extract: improves in vitro development of ovine secondary follicles |
| Gouveia et al., 2016 | Leaves | Ethanolic extract | - | Improves in vitro development of caprine preantral follicles |
| Pereira et al., 2017a | Seeds | Ethanolic extract and fractions | - | Ethanolic extracts and fractions: neuroprotective (glutamate-induced excitotoxicity) to cell lines |
| Pereira et al., 2017b | Seeds | Ethanolic extract and fractions | - | Ethanolic extracts and fractions: neuroprotective (glutamate-induced excitotoxicity) to primary culture |
| Oliveira et al., 2020 | Seeds | Protein extract and fractions | - | Synergistic antibacterial effect against S. aureus and E. coli; trypsin activity inhibitor. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.H.C.e.; Ferreira, R.S.; Pereira, E.P.; Braga-de-Souza, S.; Almeida, M.M.A.d.; Santos, C.C.d.; Butt, A.M.; Caiazzo, E.; Capasso, R.; Silva, V.D.A.d.; et al. Amburana cearensis: Pharmacological and Neuroprotective Effects of Its Compounds. Molecules 2020, 25, 3394. https://doi.org/10.3390/molecules25153394
Silva JHCe, Ferreira RS, Pereira EP, Braga-de-Souza S, Almeida MMAd, Santos CCd, Butt AM, Caiazzo E, Capasso R, Silva VDAd, et al. Amburana cearensis: Pharmacological and Neuroprotective Effects of Its Compounds. Molecules. 2020; 25(15):3394. https://doi.org/10.3390/molecules25153394
Chicago/Turabian StyleSilva, Juliana Helena Castro e, Rafael Short Ferreira, Erica Patricia Pereira, Suzana Braga-de-Souza, Monique Marylin Alves de Almeida, Cleonice Creusa dos Santos, Arthur Morgan Butt, Elisabetta Caiazzo, Raffaele Capasso, Victor Diogenes Amaral da Silva, and et al. 2020. "Amburana cearensis: Pharmacological and Neuroprotective Effects of Its Compounds" Molecules 25, no. 15: 3394. https://doi.org/10.3390/molecules25153394
APA StyleSilva, J. H. C. e., Ferreira, R. S., Pereira, E. P., Braga-de-Souza, S., Almeida, M. M. A. d., Santos, C. C. d., Butt, A. M., Caiazzo, E., Capasso, R., Silva, V. D. A. d., & Costa, S. L. (2020). Amburana cearensis: Pharmacological and Neuroprotective Effects of Its Compounds. Molecules, 25(15), 3394. https://doi.org/10.3390/molecules25153394