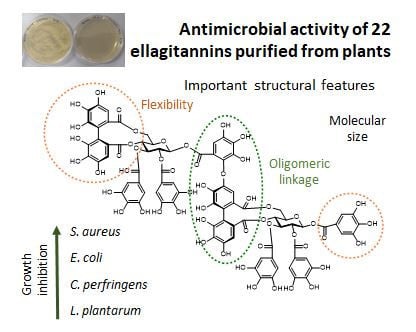
Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibitory Effects of Ellagitannins against S. aureus
2.2. Inhibitory Effects of Ellagitannins against E. coli
2.3. Inhibitory Effects of Ellagitannins against C. perfringens
2.4. Inhibitory Effects of Ellagitannins against L. plantarum
2.5. Structure–Activity Relationships
3. Materials and Methods
3.1. Tannin Selection, Isolation and Characterization
3.2. Bacterial Culture Conditions
3.3. Antimicrobial Testing of ETs
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lowry, J.; McSweeney, C.; Palmer, B. Changing perceptions of the effect of plant phenolics on nutrient supply in the ruminant. Aust. J. Agric. Res. 1996, 47, 829–842. [Google Scholar] [CrossRef]
- Min, B.; Barry, T.; Attwood, G.; McNabb, W. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Waghorn, G.; McNabb, W. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc. Nutr. Soc. 2003, 62, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop. Sci. 2019, 59, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Baert, N.; Pellikaan, W.F.; Karonen, M.; Salminen, J.-P. A study of the structure-activity relationship of oligomeric ellagitannins on ruminal fermentation in vitro. J. Dairy Sci. 2016, 99, 8041–8052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gontijo, D.C.; Gontijo, P.C.; Brandão, G.C.; Diaz, M.A.N.; de Oliveira, A.B.; Fietto, L.G.; Leite, J.P.V. Antioxidant study indicative of antibacterial and antimutagenic activities of an ellagitannin-rich aqueous extract from the leaves of Miconia latecrenata. J. Ethnopharmacol. 2019, 236, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Gupta, S.; Jacob, M.; Khan, S.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Karonen, M.; Ahern, J.R.; Legroux, L.; Suvanto, J.; Engström, M.; Sinkkonen, J.; Salminen, J.-P.; Hoste, H. Ellagitannins inhibit the exsheathment of H. contortus and T. colubriformis larvae: The efficiency increases as the molecular size increases. J. Agric. Food Chem. 2020, 68, 4176–4186. [Google Scholar]
- Salminen, J.-P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Hagerman, A.E. Fifty years of polyphenol–protein complexes. In Recent Advances in Polyphenol Research; Cheynier, V., Sarni-Manchado, P., Quideau, S., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; Volume 3, pp. 71–97. [Google Scholar]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Ellagitannins with glucopyranose cores have higher affinities to proteins than acyclic ellagitannins by isothermal titration calorimetry. J. Agric. Food Chem. 2019, 67, 12730–12740. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Karonen, M.; Ahern, J.R.; Baert, N.; Payré, B.; Hoste, H.; Salminen, J.-P. Chemical structures of plant hydrolyzable tannins reveal their in vitro activity against egg hatching and motility of Haemonchus contortus Nematodes. J. Agric. Food Chem. 2016, 64, 840–851. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez-Ortiz-De-montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hatano, T.; Ito, H.; Okuda, T. Structural diversity and antimicrobial activities of ellagitannins. In Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenolsiology Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; World Scientific Publishing: Singapore, 2009; pp. 55–93. [Google Scholar]
- Kolodziej, H.; Kayser, O.; Latté, K.P.; Kiderlen, A. Enhancement of antimicrobial activity of tannins and related compounds by immune modulatory effects. In Plant Polyphenols 2: Chemistry, Biology, Ecology; Gross, G.G., Hemingway, R.W., Yoshida, T., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 575–594. ISBN 9781461541394. [Google Scholar]
- Taguri, T.; Tanaka, T.; Kouno, I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 2006, 29, 2226–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funatogawa, K.; Hayashi, S.; Shimomura, H.; Yoshida, T.; Hatano, T.; Ito, H.; Hirai, Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol. Immunol. 2004, 48, 251–261. [Google Scholar] [CrossRef]
- Li, N.; Luo, M.; Fu, Y.; Zu, Y.; Wang, W.; Zhang, L.; Yao, L.; Zhao, C.; Sun, Y. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phyther. Res. 2013, 27, 1517–1523. [Google Scholar]
- Ranilla, L.G.; Apostolidis, E.; Shetty, K. Antimicrobial activity of an amazon medicinal plant (Chancapiedra) (Phyllanthus niruri L.) against Helicobacter pylori and lactic acid bacteria. Phyther. Res. 2012, 26, 791–799. [Google Scholar] [CrossRef]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef]
- Choe, U.; Li, Y.; Yu, L.; Gao, B.; Wang, T.T.Y.; Sun, J.; Chen, P.; Yu, L. Chemical composition of cold-pressed blackberry seed flour extract and its potential health-beneficial properties. Food Sci. Nutr. 2020, 8, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.Q.; Jin, L.; Xu, Z.J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157:H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Ossipov, V.; Loponen, J.; Haukioja, E.; Pihlaja, K. Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 1999, 864, 283–291. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal variation in the content of hydrolysable tannins in leaves of Betula pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Moilanen, J.; Salminen, J.-P. Ecologically neglected tannins and their biologically relevant activity: Chemical structures of plant ellagitannins reveal their in vitro oxidative activity at high pH. Chemoecology 2008, 18, 73–83. [Google Scholar] [CrossRef]
- Karonen, M.; Parker, J.; Agrawal, A.; Salminen, J.-P. First evidence of hexameric and heptameric ellagitannins in plants detected by liquid chromatography/electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3151–3156. [Google Scholar] [CrossRef]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Binding of an oligomeric ellagitannin series to bovine serum albumin (BSA): Analysis by isothermal titration calorimetry (ITC). J. Agric. Food Chem. 2015, 63, 10647–10654. [Google Scholar] [CrossRef]
- Virtanen, V.; Karonen, M. Partition coefficients (logP) of hydrolysable tannins. Molecules 2020, 25, 3691. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Wolfender, J.-L.; Salminen, J.-P.; Pihlaja, K.; Hostettmann, K.; Vuorela, H. Characterization of the polyphenolic composition of purple loosestrife (Lythrum salicaria). Zeitschrift Naturforsch. Sect. C J. Biosci. 2001, 56, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J. Chem. Ecol. 2004, 30, 1693–1711. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Yarnes, C.T.; Boecklen, W.J.; Tuominen, K.; Salminen, J.-P. Defining phytochemical phenotypes: Size and shape analysis of phenolic compounds in oaks (Fagaceae, Quercus) of the Chihuahuan Desert. Can. J. Bot. 2006, 84, 1233–1248. [Google Scholar] [CrossRef]
- Yarnes, C.T.; Boecklen, W.J.; Salminen, J.-P. No simple sum: Seasonal variation in tannin phenotypes and leaf-miners in hybrid oaks. Chemoecology 2008, 18, 39–51. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, K.; Chen, X.; Okuda, T. Tannins from Hippophae rhamnoides. Phytochemistry 1991, 30, 663–666. [Google Scholar] [CrossRef]
- Suvanto, J.; Tähtinen, P.; Valkamaa, S.; Engström, M.T.; Karonen, M.; Salminen, J.-P. Variability in foliar ellagitannins of Hippophaë rhamnoides L. and identification of a new ellagitannin, hippophaenin C. J. Agric. Food Chem. 2018, 66, 613–620. [Google Scholar] [CrossRef]
- Matsuo, Y.; Wakamatsu, H.; Omar, M.; Tanaka, T. Reinvestigation of the stereochemistry of the C-glycosidic ellagitannins, vescalagin and castalagin. Org. Lett. 2015, 17, 46–49. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Yazaki, K.; Ashida, M. Ellagitannins of the casuarinaceae, stachyuraceae and myrtaceae. Phytochemistry 1980, 21, 2871–2874. [Google Scholar] [CrossRef]
- Tuominen, A.; Toivonen, E.; Mutikainen, P.; Salminen, J.-P. Defensive strategies in Geranium sylvaticum. Part 1: Organ-specific distribution of water-soluble tannins, flavonoids and phenolic acids. Phytochemistry 2013, 95, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Yokota, T.; Lean, M.E.J.; Crozier, A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Moilanen, J.; Koskinen, P.; Salminen, J.-P. Distribution and content of ellagitannins in finnish plant species. Phytochemistry 2015, 116, 188–197. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Iwasaki, M.; Kubo, M.; Orime, T.; Yoshizaki, M.; Naruhashi, N. Hydrolysable tannins as chemotaxonomic markers in the rosaceae. Phytochemistry 1992, 31, 3091–3096. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Kiss, A.K. C-glucosidic ellagitannins from lythri herba (European Pharmacopoeia): Chromatographic profile and structure determination. Phytochem. Anal. 2013, 24, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Karatzas, K.A.G.; Zervos, A.; Tassou, C.C.; Mallidis, C.G.; Humphrey, T.J. Piezotolerant small-colony variants with increased thermotolerance, antibiotic susceptibility, and low invasiveness in a clonal Staphylococcus aureus population. Appl. Environ. Microbiol. 2007, 73, 1873–1881. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. https://doi.org/10.3390/molecules25163714
Puljula E, Walton G, Woodward MJ, Karonen M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules. 2020; 25(16):3714. https://doi.org/10.3390/molecules25163714
Chicago/Turabian StylePuljula, Elina, Gemma Walton, Martin J. Woodward, and Maarit Karonen. 2020. "Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus" Molecules 25, no. 16: 3714. https://doi.org/10.3390/molecules25163714
APA StylePuljula, E., Walton, G., Woodward, M. J., & Karonen, M. (2020). Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules, 25(16), 3714. https://doi.org/10.3390/molecules25163714