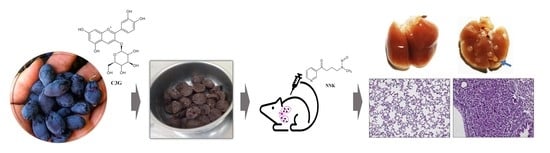
Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice
Abstract
:1. Introduction
2. Results
2.1. The Composition of C3G-HB
2.2. The Response of Mice to C3G-HB Supplementation and NNK-Injection
2.3. Lung Tumorigenesis and Tumor Incidence
2.4. Lung Tumor Area
2.5. Expression of PCNA and Ki-67
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of C3G-HB and Analysis
4.3. Preparation of Dietary Supplement
4.4. Experimental Plan and Procedure
4.5. Lung Tumor Assays
4.5.1. Positron Emission Tomography-Magnetic Resonance Imaging (PET-MRI)
4.5.2. Tumor Histology and Tumor Area
4.5.3. Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Hecht, S.S. It Is Time to Regulate Carcinogenic Tobacco-Specific Nitrosamines in Cigarette Tobacco. Cancer Prev. Res. 2014, 7, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Hecht, S.S. Tobacco Smoke Carcinogens and Lung Cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef] [Green Version]
- Ronai, Z.A.; Gradia, S.; Peterson, L.A.; Hecht, S.S. SHORT COMMUNICATION: G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and relati DNA methylating and pyridyloxobutylating agents. Carcinogenesis 1993, 14, 2419–2422. [Google Scholar] [CrossRef]
- Yamakawa, K.; Yokohira, M.; Nakano, Y.; Kishi, S.; Kanie, S.; Imaida, K. Activation of MEK1/2-ERK1/2 signaling during NNK-induced lung carcinogenesis in female A/J mice. Cancer Med. 2016, 5, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.M.; Wheeler, R.; Singh, N.; Vosloo, A.; Ray, D.W.; Sommer, P. The tobacco carcinogen NNK drives accumulation of DNMT1 at the GR promoter thereby reducing GR expression in untransformed lung fibroblasts. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-Y.; Li, M.-Y.; Hsin, M.K.Y.; Underwood, M.J.; Ma, L.T.; Mok, T.S.-K.; Warner, T.D.; Chen, G.G. 4-Methylnitrosamino-1-3-pyridyl-1-butanone (NNK) promotes lung cancer cell survival by stimulating thromboxane A2 and its receptor. Oncogene 2010, 30, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Amararathna, M.; Johnston, M.R.; Rupasinghe, H.P.V. Plant Polyphenols as Chemopreventive Agents for Lung Cancer. Int. J. Mol. Sci. 2016, 17, 1352. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Afaq, F.; Kweon, M.-H.; Kim, K.; Mukhtar, H. Oral Consumption of Pomegranate Fruit Extract Inhibits Growth and Progression of Primary Lung Tumors in Mice. Cancer Res. 2007, 67, 3475–3482. [Google Scholar] [CrossRef] [Green Version]
- Charepalli, V.; Reddivari, L.; Radhakrishnnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K.P. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Liang, K.; De Mejia, E.G. Inhibitory potential of anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- He, Y.; Hu, Y.; Jiang, X.; Chen, T.; Ma, Y.; Wu, S.; Sun, J.; Jiao, R.; Li, X.; Deng, L.; et al. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells. J. Photochem. Photobiol. B Biol. 2017, 177, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Yu, L.J.; Bhullar, K.S.; Bors, B. Short Communication: Haskap (Lonicera caerulea): A new berry crop with high antioxidant capacity. Can. J. Plant Sci. 2012, 92, 1311–1317. [Google Scholar] [CrossRef]
- Khattab, R.; Brooks, M.S.-L.; Ghanem, A. Phenolic Analyses of Haskap Berries (Lonicera caerulea L.): Spectrophotometry Versus High Performance Liquid Chromatography. Int. J. Food Prop. 2015, 19, 1708–1725. [Google Scholar] [CrossRef] [Green Version]
- Rupasinghe, H.P.V.; Boehm, M.M.A.; Sekhon-Loodu, S.; Parmar, I.; Bors, B.; Jamieson, A.R. Anti-Inflammatory Activity of Haskap Cultivars is Polyphenols-Dependent. Biomolecules 2015, 5, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Ma, Y.; Wang, X.; Zhang, X.; Zhang, Q.; Meng, X. Lonicera caerulea berry extract attenuates lipopolysaccharide induced inflammation in BRL-3A cells: Oxidative stress, energy metabolism, hepatic function. J. Funct. Foods 2016, 24, 1–10. [Google Scholar] [CrossRef]
- Wu, S.; He, X.; Wu, X.; Qin, S.; He, J.; Zhang, S.; Hou, D.-X. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: Crosstalk of anti-inflammatory and antioxidant effects. J. Funct. Foods 2015, 17, 514–523. [Google Scholar] [CrossRef]
- Liu, M.; Tan, J.; He, Z.; He, X.; Hou, D.-X.; He, J.; Wu, S. Inhibitory effect of blue honeysuckle extract on high-fat-diet-induced fatty liver in mice. Anim. Nutr. 2018, 4, 288–293. [Google Scholar] [CrossRef]
- De Silva, A.K.H.; Rupasinghe, H.P.V.; Kithma, A. Polyphenols composition and anti-diabetic properties in vitro of haskap (Lonicera caerulea L.) berries in relation to cultivar and harvesting date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Amararathna, M.; Hoskin, D.; Rupasinghe, H.P.V. Anthocyanin-rich haskap (Lonicera caerulea L.) berry extracts reduce nitrosamine-induced DNA damage in human normal lung epithelial cells. Food Chem. Toxicol. 2020, 141, 11140. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.-Z.; Xu, T.-R.; Chen, C.-S. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim. Biophys. Sin. 2015, 47, 477–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeidler-Erdely, P.C.; Kashon, M.L.; Battelli, L.A.; Young, S.-H.; Erdely, A.; Roberts, J.R.; Reynolds, S.H.; Antonini, J.M. Pulmonary inflammation and tumor induction in lung tumor susceptible A/J and resistant C57BL/6J mice exposed to welding fume. Part. Fibre Toxicol. 2008, 5, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, S.S.; Isaacs, S.; Trushin, N. Lung tumor induction in A/J mice by the tobacco smoke carcinogens 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone and benzo[a]pyrene: A potentially useful model for evaluation of chemopreventive agents. Carcinogenesis 1994, 15, 2721–2725. [Google Scholar] [CrossRef]
- Maser, E.; Richter, E.; Friebertshäuser, J. The Identification of 11beta-hydroxysteroid Dehydrogenase as Carbonyl Reductase of the Tobacco-Specific Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Eur. J. Biochem. 1996, 238, 484–489. [Google Scholar] [CrossRef]
- Hecht, S.S.; Trushin, N.; Reid-Quinn, C.A.; Burak, E.S.; Jones, A.B.; Southers, J.L.; Gombar, C.T.; Carmella, S.G.; Anderson, L.M.; Rice, J.M. Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the patas monkey: Pharmacokinetics and characterization of glucuronide metabolites. Carcinogenesis 1993, 14, 229–236. [Google Scholar] [CrossRef]
- Svobodová, A.R.; Galandáková, A.; Palikova, I.; Dolezal, D.; Kylarova, D.; Ulrichová, J.; Vostálová, J. Effects of oral administration of Lonicera caerulea berries on UVB-induced damage in SKH-1 mice. A pilot study. Photochem. Photobiol. Sci. 2013, 12, 1830. [Google Scholar] [CrossRef]
- Vostálová, J.; Galandáková, A.; Palikova, I.; Ulrichová, J.; Dolezal, D.; Lichnovska, R.; Vrbkova, J.; Svobodová, A.R. Lonicera caerulea fruits reduce UVA-induced damage in hairless mice. J. Photochem. Photobiol. B Biol. 2013, 128, 1–11. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.-Y.; Ma, F.; Yang, X.; Cheng, C.; Yao, L. Protective Effect of Anthocyanin from Lonicera Caerulea var. Edulis on Radiation-Induced Damage in Mice. Int. J. Mol. Sci. 2012, 13, 11773–11782. [Google Scholar] [CrossRef]
- Bojić, M.; Kondža, M.; Rimac, H.; Benković, G.; Males, Z. The Effect of Flavonoid Aglycones on the CYP1A2, CYP2A6, CYP2C8 and CYP2D6 Enzymes Activity. Molecules 2019, 24, 3174. [Google Scholar] [CrossRef] [Green Version]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Y.; Yuan, X.; Liu, X.; Liang, C.; Meng, M.; Huang, Y.; Han, X.; Guo, J.; Ren, C.; Zhang, Q.; et al. Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function. Mol. Nutr. Food Res. 2017, 61, 1–13. [Google Scholar]
- Titta, L.; Trinei, M.; Stendardo, M.; Berniakovich, I.; Petroni, K.; Tonelli, C.; Riso, P.; Porrini, M.; Minucci, S.; Pelicci, P.G.; et al. Blood orange juice inhibits fat accumulation in mice. Int. J. Obes. 2009, 34, 578–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.-H.; Jung, K.O.; Kwon, K.-I.; Jeong, T.C.; Chung, Y.C.; Jeong, H.G.; et al. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate–activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906. [Google Scholar] [CrossRef]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B.-J. Targeting AMPK for cancer prevention and treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [Green Version]
- Marczylo, T.H.; Cooke, D.; Brown, K.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and metabolism of the putative cancer chemopreventive agent cyanidin-3-glucoside in mice. Cancer Chemother. Pharmacol. 2009, 64, 1261–1268. [Google Scholar] [CrossRef]
- Fornasaro, S.; Ziberna, L.; Gasperotti, M.; Tramer, F.; Vrhovšek, U.; Mattivi, F.; Passamonti, S. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci. Rep. 2016, 6, 22815. [Google Scholar] [CrossRef]
- Felgines, C.; Krisa, S.; Mauray, A.; Besson, C.; Lamaison, J.-L.; Scalbert, A.; Mérillon, J.-M.; Texier, O. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. Br. J. Nutr. 2010, 103, 1738–1745. [Google Scholar] [CrossRef] [Green Version]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated Dietary Polyphenol Intake and Major Food and Beverage Sources among Elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Lonair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: C3G-HB is available from the authors for collaborative research. |





| Nutrient | % | Mineral Content | % |
|---|---|---|---|
| Dry matter | 91.64 | Potassium | 1.175 |
| Protein digestibility | 67.76 | Magnesium | 0.056 |
| Crude protein | 4.86 | Phosphorous | 0.176 |
| Bound protein | 5.10 | Calcium | 0.105 |
| ADIN | 0.25 | Sodium | 0.016 |
| Crude fat | 3.33 | Copper (mg/kg) | 6.34 |
| Acid detergent fiber | 3.64 | Manganese (mg/kg) | <10.00 |
| Neutral detergent fiber | 4.28 | Zinc (mg/kg) | 7.74 |
| Ash | 2.57 | Cyanidin-3-O-glucoside | 3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice. Molecules 2020, 25, 3823. https://doi.org/10.3390/molecules25173823
Amararathna M, Hoskin DW, Rupasinghe HPV. Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice. Molecules. 2020; 25(17):3823. https://doi.org/10.3390/molecules25173823
Chicago/Turabian StyleAmararathna, Madumani, David W. Hoskin, and H. P. Vasantha Rupasinghe. 2020. "Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice" Molecules 25, no. 17: 3823. https://doi.org/10.3390/molecules25173823
APA StyleAmararathna, M., Hoskin, D. W., & Rupasinghe, H. P. V. (2020). Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice. Molecules, 25(17), 3823. https://doi.org/10.3390/molecules25173823