Characteristics of Polyphenolic Content in Brown Algae of the Pacific Coast of Russia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Polyphenolic Contents of the Algae Water and Ethanol Extracts
2.2. Phlorotannins Content in Algae Water and Alcohol Extracts
2.3. Determination of the Antioxidan Activity of the Algae Water and Ethanol Extracts
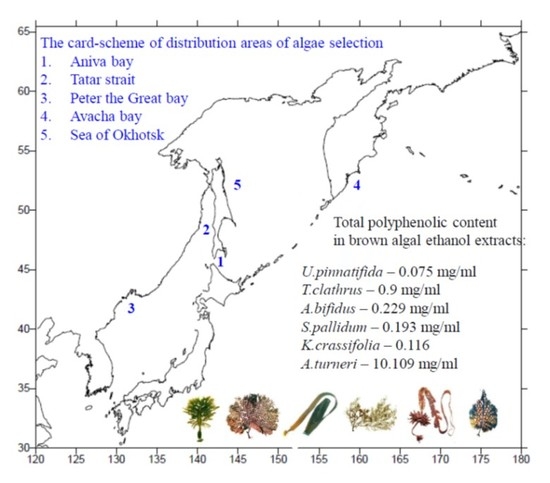
2.4. Correlation between Radical Scavenging Activity, Phenolic Content and Habitat Area of Brown Algae
2.5. Isolation and Identification of Phenolic Compounds from T. clathrus
3. Materials and Methods
3.1. Algal Materials
3.2. Determination of Total Polyphenolics
3.3. Determination of Total Phlorotannins Content
3.4. 2,2-Diphenyl-1-Picrylhydrazine Radical Scavenging Activity
3.5. 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid) Radical Scavenging Activity
3.6. UV-Visible Spectroscopy
3.7. High Performance Liquid Chromatography of Polyphenols
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal metabolites: An inevitable substitute for antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, V.T.; Hung, N.D.; Se-Kwon, K. Pharmaceutical properties of marine polyphenols: An overview. Acta Pharm. Sci. 2019, 52, 217–242. [Google Scholar] [CrossRef] [Green Version]
- Koivikko, R. Brown Algal Phlorotannins: Improving and Applying Chemical Methods; Painosalama Oy: Turku, Finland, 2008. [Google Scholar]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Druzhinina, A.; Kaplitsin, P.; Ovchinnikov, D.; Parshina, A.; Kuznetsova, M. Relationship between radical scavenging activity and polymolecular properties of brown algae polyphenols. Chem. Pap. 2019, 73, 2377–2385. [Google Scholar] [CrossRef]
- Vega, J.Á.-G.F.; Güenaga, L.; Figueroa, F.L.; Gómez-Pinchetti, J.L. Antioxidant activity of extracts from marine macroalgae, wild-collected and cultivated, in an integrated multi-trophic aquaculture system. Aquaculture 2020, 522, 1–10. [Google Scholar] [CrossRef]
- Santoso, J.; Yoshie, Y.; Suzuki, T. The distribution and profile of nutrients and catechins of some Indonesian seaweeds. Fish. Sci. 2002, 68, 1647–1648. [Google Scholar] [CrossRef] [Green Version]
- Yoshie-Stark, Y. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Barbosa, M.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, Á.; Andrade, P.B. In vitro multifunctionality of phlorotannin extracts from edible Fucus species on targets underpinning neurodegeneration. Food Chem. 2020, 333, 127456. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. An emerging trend in functional foods for the prevention of cardiovascular disease and diabetes: Marine algal polyphenols. Crit. Rev. Food Sci. Nutr. 2018, 58, 1342–1358. [Google Scholar] [CrossRef] [PubMed]
- Bupesh, G.; Amutha, C.; Kumar, A.G.; Vasanth, S.; Surendiran, L.; Balachandar, V. Prospective liver shielding activity of marine red algae on aflatoxin-induced toxicity in rats. Drug Invent. Today 2019, 11, 2633–2636. [Google Scholar]
- Kim, Y.C.; An, R.B.; Yoon, N.Y.; Nam, T.J.; Choi, J.S. Hepatoprotective constituents of the edible brown alga Ecklonia stolonifera on tacrine-induced cytotoxicity in Hep G2 cells. Arch. Pharmacal Res. 2005, 28, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, K.; Thirugnanasambandan, S.S. Polyphenols from brown alga, Padina boergesenii (Allendar & Kraft) decelerates renal cancer growth involving cell cycle arrest and induction of apoptosis in renal carcinoma cells. Environ. Toxicol. 2018, 33, 1135–1142. [Google Scholar] [PubMed]
- Fernando, I.P.S.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa, C.R.; Barriga, A.; Maki-Arvela, P.; Buschmann, A.H. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Dobrodeeva, L.; Druzhinina, A.; Ovchinnikov, D.; Parshina, A.; Shulgina, E. Biological activity of a polyphenolic complex of Arctic brown algae. J. Appl. Phycol. 2019, 31, 3341–3348. [Google Scholar] [CrossRef]
- Lee, R.E. Phycology, 4th ed.; Journal of Botany: Edinburgh, UK, 2009; Volume 66, pp. 483–484. [Google Scholar]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentao, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 6, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Coimbra, Portugal, 2016. [Google Scholar]
- Vilkova, O. The place of Russia in the world seaweed production. Rybprom 2010, 3, 4–8. [Google Scholar]
- Suhoveeva, M.; Podkorytova, A. Commercial Seaweed and Grass of the Far East Seas: Biology, Distribution, Stocks, Processing Technology; TINRO: Vladivostok, Russia, 2006. [Google Scholar]
- Ragan, M.; Glombitza, K. Phlorotannins, brown algal polyphenols. In Progress in Phycological Research; Round, F.E., Chapman, D.J., Eds.; Biopress: Devon, UK, 1986; Volume 4, pp. 129–241. [Google Scholar]
- Nisizawa, K. Seaweeds Kaiso; Japan Seaweed Association: Tosa, Kochi, Japan, 2002. [Google Scholar]
- Fernald, M.L.; Kinsey, A.C.; Rollins, R.C. Wild Plants of Eastern North America, 2nd ed.; Harper: New York, NY, USA, 1958. [Google Scholar]
- Titlyanov, E.; Titlyanova, T. Marine Plants of Asian-Pacific Region, Their Use and Cultivation; Dal’nauka: Vladivostok, Russia, 2012. [Google Scholar]
- Li, Y.J.; Fu, X.T.; Duan, D.L.; Liu, X.Y.; Xu, J.C.; Gao, X. Extraction and identification of phlorotannins from the brown alga, sargassum fusiforme (Harvey) setchell. Mar. Drugs 2017, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yuan, W.; Meng, X. Extraction and quantification of phlorotannins from edible brown algae. Trans. Asabe 2017, 60, 265–271. [Google Scholar]
- Barton, A.F. Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Coimbra, Portugal, 1983. [Google Scholar]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Farvin, K.H.S.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Aminina, N.M.; Vishnevskaya, T.I.; Karaulova, E.P.; Yakush, E.V. Content of polyphenols and antioxidant activity of extracts from certain species of seaweeds. Izv. TINRO 2017, 189, 184–191. [Google Scholar]
- Koivikko, R.; Eranen, J.K.; Loponen, J.; Jormalainen, V. Variation of phlorotannins among three populations of Fucus vesiculosus as revealed by HPLC and colorimetric quantification. J. Chem. Ecol. 2008, 34, 57–64. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.-C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome Okamura, and their radical scavenging activities. Mar. Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Stern, J.L.; Hagerman, A.E.; Steinberg, P.D.; Winter, F.C.; Estes, J.A. A new assay for quantifying brown algal phlorotannins and comparisons to previous methods. J. Chem. Ecol. 1996, 22, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; González, M.C. Optimization strategy and validation of one chromatographic method as approach to determine the phenolic compounds from different sources. J. Chromatogr. A 2000, 897, 161–170. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
 ) extracts and ethanol (
) extracts and ethanol (  ) extracts. Right axis—date for A. turneri.
) extracts. Right axis—date for A. turneri.
 ) extracts and ethanol (
) extracts and ethanol (  ) extracts. Right axis—date for A. turneri.
) extracts. Right axis—date for A. turneri.
 ) and ethanol (
) and ethanol (  ) extracts. Right axis—date for A. turneri.
) extracts. Right axis—date for A. turneri.
 ) and ethanol (
) and ethanol (  ) extracts. Right axis—date for A. turneri.
) extracts. Right axis—date for A. turneri.



| Family | Specie | Site of Algae Selection | Commercial Use of Algae | |
|---|---|---|---|---|
| Laminariaceae | Laminaria cichorioides = Saccharina cichorioides | Sea of Japan | Aniva Bay | Food product [24] |
| Laminariaceae | Laminaria bongardiana = Saccharina bongardiana | Pacific Ocean | Avacha Bay | Food product [25,26] |
| Laminariaceae | Kjellmaniella crassifolia = Saccharina sculpera | Sea of Okhotsk | Spaseniya Bay | Food product [27] |
| Alariaceae | Undaria pinnatifida | Sea of Japan | Peter the Great Bay | Food product [28] |
| Alariaceae | Alaria angusta | Pacific Ocean | Spaseniya Bay | Potentially commercial [26] |
| Arthrothamnaceae | Arthrothamnus bifidus | Pacific Ocean | Avacha Bay | Food product [27] |
| Costariaceae | Costaria costata | Sea of Japan | Tatar Strait | Potentially commercial [26] |
| Costariaceae | Thalassiophyllum clathrus | Pacific Ocean | Avacha Bay | Potentially commercial [26] |
| Costariaceae | Agarum turneri | Pacific Ocean | Avacha Bay | Food product [29] |
| Sargassaceae | Sargassum miyabei | Sea of Japan | Peter the Great Bay | Potentially commercial [26]; food product [24] |
| Sargassaceae | Sargassum pallidum | Sea of Japan | Peter the Great Bay | Food product [28] |
| Cystoseiraceae | Cystoseira crassipes = Stephanocystis crassipes | Sea of Okhotsk | Aniva Bay | Potentially commercial [26] |
| Fucaceae | Fucus evanescens | Sea of Okhotsk | Aniva Bay | Food product [30] |
| Description | Radical Scavenging Activity, Ethanol Extract | Radical Scavenging Activity, Water Extract | ||
|---|---|---|---|---|
| DPPH, mg Ascorbic Acid/g Dry Algae | ABTS, µmol Trolox Equiv/g Dry Algae | DPPH, mg Ascorbic Acid/g Dry Algae | ABTS, µmol Trolox Equiv/g Dry Algae | |
| Laminaria cichorioides | 0.3 ± 0.02 | 15.2 ± 1.1 | 0.1 ± 0.03 | 7.9 ± 0.9 |
| Laminaria bongardiana | 0.4 ± 0.03 | 17.4 ± 1.0 | 0.03 ± 0.02 | 2.4 ± 0.3 |
| Kjellmaniella crassifolia | 0.5 ± 0.02 | 31.6 ± 2.0 | 0.4 ± 0.02 | 16.4 ± 1.9 |
| Undaria pinnatifida | 0.3 ± 0.02 | 17.5 ± 1.1 | 0.2 ± 0.02 | 10.2 ± 0.9 |
| Alaria angusta | 0.2 ± 0.01 | 12.2 ± 1.3 | 0.2 ± 0.01 | 13.6 ± 1.2 |
| Arthrothamnus bifidus | 1.1 ± 0.05 | 64.7 ± 3.5 | 0.5 ± 0.01 | 32.6 ± 2.2 |
| Costaria costata | 0.3 ± 0.01 | 18.2 ± 1.8 | 0.8 ± 0.01 | 46.9 ± 2.5 |
| Thalassiophyllum clathrus | 2.2 ± 0.05 | 137.0 ± 2.9 | 1.4 ± 0.1 | 82.5 ± 2.8 |
| Agarum turneri | 38.8 ± 2.4 | 2506.8 ± 95.6 | 16.7 ± 2.4 | 1026.3 ± 96.1 |
| Sargassum miyabei | 1.1 ± 0.05 | 68.7 ± 2.3 | 0.8 ± 0.03 | 38.6 ± 2.5 |
| Sargassum pallidum | 1.2 ± 0.1 | 68.9 ± 3.2 | 1.4 ± 0.05 | 75.3 ± 3.4 |
| Cystoseira crassipes | 2.3 ± 0.1 | 116.1 ± 3.4 | 1.1 ± 0.05 | 65.3 ± 2.1 |
| Fucus evanescens | 4.5 ± 0.1 | 291.9 ± 5.6 | 1.2 ± 0.1 | 85.3 ± 3.1 |
| Phenolic Compound | Rt ± SD, min | UV Bands, nm |
|---|---|---|
| Gallic acid | 9.75 ± 0.02 | 270 |
| (+)-Catechin | 15.14 ± 0.07 | 280 |
| Chlorogenic acid | 18.95 ± 0.04 | 240; 325 |
| (−)-Epicatechin | 22.95 ± 0.10 | 280 |
| Caffeic acid | 28.46 ± 0.09 | 240; 325 |
| Coumaric acid | 35.32 ± 0.10 | 225; 310 |
| Rutin | 45.82 ± 0.11 | 255; 355 |
| Quercetin | 56.30 ± 0.05 | 250; 368 |
| Apigenin | 76.46 ± 0.08 | 265; 335 |
| Kaempferol | 77.85 ± 0.07 | 260; 367 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminina, N.M.; Karaulova, E.P.; Vishnevskaya, T.I.; Yakush, E.V.; Kim, Y.-K.; Nam, K.-H.; Son, K.-T. Characteristics of Polyphenolic Content in Brown Algae of the Pacific Coast of Russia. Molecules 2020, 25, 3909. https://doi.org/10.3390/molecules25173909
Aminina NM, Karaulova EP, Vishnevskaya TI, Yakush EV, Kim Y-K, Nam K-H, Son K-T. Characteristics of Polyphenolic Content in Brown Algae of the Pacific Coast of Russia. Molecules. 2020; 25(17):3909. https://doi.org/10.3390/molecules25173909
Chicago/Turabian StyleAminina, Natalia M., Ekaterina P. Karaulova, Tatiana I. Vishnevskaya, Evgeny V. Yakush, Yeon-Kye Kim, Ki-Ho Nam, and Kwang-Tae Son. 2020. "Characteristics of Polyphenolic Content in Brown Algae of the Pacific Coast of Russia" Molecules 25, no. 17: 3909. https://doi.org/10.3390/molecules25173909
APA StyleAminina, N. M., Karaulova, E. P., Vishnevskaya, T. I., Yakush, E. V., Kim, Y. -K., Nam, K. -H., & Son, K. -T. (2020). Characteristics of Polyphenolic Content in Brown Algae of the Pacific Coast of Russia. Molecules, 25(17), 3909. https://doi.org/10.3390/molecules25173909