Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials
Abstract
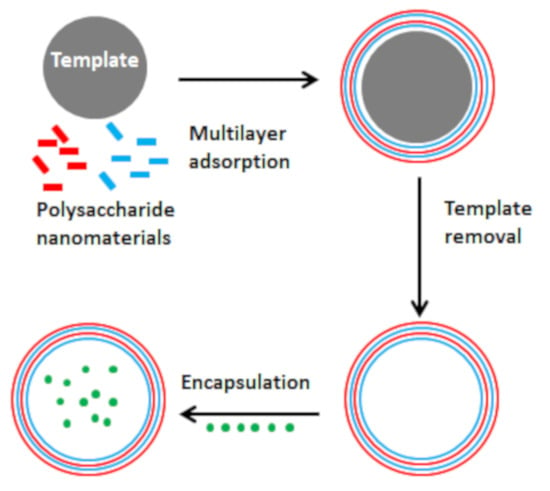
:1. Introduction
2. Polysaccharide Nanomaterials
2.1. Cellulose Nanomaterials
2.2. Chitin Nanomaterials
2.3. Starch Nanomaterials
3. Methods of Microcapsule Fabrication
4. Microcapsules Based on Polysaccharide Nanomaterials
4.1. Ion Cross-Linked Polysaccharide Microcapsules Reinforced with Polysaccharide Nanomaterials
4.2. Biomimetic Polysaccharide Nanomaterials Microcapsules Stabilized by Electrostatic and H-Bond Interactions
4.3. Microcapsules Prepared by In Situ Core Formation Stabilized by Polysaccharide Nanomaterials
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bah, M.G.; Bilal, H.M.; Wang, J. Fabrication and application of complex microcapsules: A review. Soft Matter 2020, 16, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A Review of Applications in the Food and Pharmaceutical Industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.I.; Ferreira, I.C.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [Green Version]
- Corrêa-Filho, L.; Moldão-Martins, M.; Alves, V. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application--a review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef]
- Hu, X.P.; Shasha, B.S.; McGuire, M.R.; Prokopy, R.J. Controlled release of sugar and toxicant from a novel device for controlling pest insects. J. Control. Release 1998, 50, 257–265. [Google Scholar] [CrossRef]
- Han, N.X.; Xing, F. A Comprehensive Review of the Study and Development of Microcapsule Based Self-Resilience Systems for Concrete Structures at Shenzhen University. Materials 2016, 10. [Google Scholar] [CrossRef]
- Urbas, R.; Milošević, R.; Kašiković, N.; Pavlović, Ž.; Elesini, U.S. Microcapsules application in graphic arts industry: A review on the state-of-the-art. Iran. Polym. J. 2017, 26, 541–561. [Google Scholar] [CrossRef]
- Jia, Y.; Feng, X.; Li, J. Polysaccharides-Based Microcapsules. In Supramolecular Chemistry of Biomimetic Systems; Li, J., Ed.; Springer Singapore: Singapore, 2017; pp. 63–84. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sukhorukov, G.B.; Möhwald, H. The pros and cons of polyelectrolyte capsules in drug delivery. Expert Opin. Drug Deliv. 2009, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, H.; Schroën, K.; Boom, R. Biodegradable polymeric microcapsules: Preparation and properties. Chem. Eng. J. 2011, 169, 1–10. [Google Scholar] [CrossRef]
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Feng, X.; Du, C.; Li, J. Molecular Assembly of Polysaccharide-Based Microcapsules and Their Biomedical Applications. Chem. Rec. 2016, 16, 1991–2004. [Google Scholar] [CrossRef]
- Carrick, C.; Ruda, M.; Pettersson, B.; Larsson, P.T.; Wågberg, L. Hollow cellulose capsules from CO2 saturated cellulose solutions—their preparation and characterization. RSC Adv. 2013, 3, 2462. [Google Scholar] [CrossRef]
- Carrick, C.; Larsson, P.A.; Brismar, H.; Aidun, C.; Wågberg, L. Native and functionalized micrometre-sized cellulose capsules prepared by microfluidic flow focusing. RSC Adv. 2014, 4, 19061–19067. [Google Scholar] [CrossRef]
- Edgar, K.J. Cellulose esters in drug delivery. Cellulose 2006, 14, 49–64. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Jewitt, T.S.; Horie, C.V. The long-term stability of cellulose ester coatings. Polym. Degrad. Stab. 1989, 26, 221–229. [Google Scholar] [CrossRef]
- Hussain, S.A.; Abdelkader, H.; Abdullah, N.; Kmaruddin, S. Review on micro-encapsulation with Chitosan for pharmaceuticals applications. MOJ Curr. Res. Rev. 2018, 1, 77–84. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, J.Z.; Malaki Nik, A. Chapter 17—Starch-Based Microencapsulation. In Starch in Food, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Swaston, UK, 2018; pp. 661–690. [Google Scholar] [CrossRef]
- Lei, W.; Fang, C.; Zhou, X.; Yin, Q.; Pan, S.; Yang, R.; Liu, D.; Ouyang, Y. Cellulose nanocrystals obtained from office waste paper and their potential application in PET packing materials. Carbohydr. Polym. 2018, 181, 376–385. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kochhar, T.; Sood, A.; Singh, B. Conversion of Potato Starch and Peel Waste to High Value Nanocrystals. Potato Res. 2018, 61, 341–351. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Wang, J.; Guan, A.; Naguib, H.E. Chitin nano-whiskers (CNWs) as a bio-based bio-degradable reinforcement for epoxy: Evaluation of the impact of CNWs on the morphological, fracture, mechanical, dynamic mechanical, and thermal characteristics of DGEBA epoxy resin. RSC Adv. 2019, 9, 11063–11076. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Clancy, A.J.; Kontturi, E.; Bismarck, A.; Shaffer, M.S. Strong and Stiff: High-Performance Cellulose Nanocrystal/Poly(vinyl alcohol) Composite Fibers. ACS Appl. Mater. Interfaces 2016, 8, 31500–31504. [Google Scholar] [CrossRef] [Green Version]
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Roth, S.V.; Lundell, F.; Wagberg, L.; Kotov, N.A.; et al. Multiscale Control of Nanocellulose Assembly: Transferring Remarkable Nanoscale Fibril Mechanics to Macroscale Fibers. ACS Nano 2018, 12, 6378–6388. [Google Scholar] [CrossRef]
- Carsi, M.; Sanchis, M.J.; Gómez, C.M.; Rodriguez, S.; G Torres, F. Effect of Chitin Whiskers on the Molecular Dynamics of Carrageenan-Based Nanocomposites. Polymers 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part. B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Kamel, S.; A Khattab, T. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Rehan, M.; Okla, M.K.; Alamri, S.A.; Alaraidh, I.A.; Al-ghamdi, A.A.; Soufan, W.H.; Abdelsalam, E.M.; Allam, A.A. Novel halochromic cellulose nanowhiskers from rice straw: Visual detection of urea. Carbohydr. Polym. 2020, 231, 115740. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F.; Xu, W.; Willför, S.; Syverud, K.; Xu, C. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D printing of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- López-López, E.A.; Hernández-Gallegos, M.A.; Cornejo-Mazón, M.; Hernández-Sánchez, H. Polysaccharide-Based Nanoparticles. In Food Nanoscience and Nanotechnology; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002. [Google Scholar] [CrossRef]
- Gao, K.; Shao, Z.; Li, J.; Wang, X.; Peng, X.; Wang, W.; Wang, F. Cellulose nanofiber–graphene all solid-state flexible supercapacitors. J. Mater. Chem. A 2013, 1, 63–67. [Google Scholar] [CrossRef]
- Goodrich, J.D.; Winter, W.T. α-Chitin Nanocrystals Prepared from Shrimp Shells and Their Specific Surface Area Measurement. Biomacromolecules 2007, 8, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Wang, S.T.; Chang, K.B.; Tsai, M.L. Enhancing Saltiness Perception Using Chitin Nanomaterials. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tian, H.; Zhang, L. Role of starch nanocrystals and cellulose whiskers in synergistic reinforcement of waterborne polyurethane. Carbohydr. Polym. 2010, 80, 665–671. [Google Scholar] [CrossRef]
- Melida, H.; Sandoval-Sierra, J.V.; Dieguez-Uribeondo, J.; Bulone, V. Analyses of extracellular carbohydrates in oomycetes unveil the existence of three different cell wall types. Eukaryot. Cell 2013, 12, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Ek, R.; Gustafsson, C.; Nutt, A.; Iversen, T.; Nyström, C. Cellulose powder from Cladophora sp. algae. J. Mol. Recognit. 1998, 11, 263–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Lindström, M.E.; Li, J. Tunicate cellulose nanocrystals: Preparation, neat films and nanocomposite films with glucomannans. Carbohydr. Polym. 2015, 117, 286–296. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [Green Version]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Walter, N.M. Liquid Crystal Systems from fibrillar Polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Stef-Mincea, M.; Negrulescu, A.; Ostafe, V. Preparation, modification, and applications of chitin nanowhiskers: A review. Rev. Adv. Mater. Sci. 2012, 30, 225–242. [Google Scholar]
- Li, J.; Revol, J.F.; Marchessault, R.H. Effect of degree of deacetylation of chitin on the properties of chitin crystallites. J. Appl. Polym. Sci. 1997, 65, 373–380. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Chitin Nanocrystals Prepared by TEMPO-Mediated Oxidation of α-Chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Barkhordari, M.R.; Fathi, M. Production and characterization of chitin nanocrystals from prawn shell and their application for stabilization of Pickering emulsions. Food Hydrocoll. 2018, 82, 338–345. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Gedanken, A. Eco-Friendly and Facile Preparation of Spherical Chitin Nanoparticles. ChemistrySelect 2018, 3, 10787–10791. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Le Corre, D.; Angellier-Coussy, H. Preparation and application of starch nanoparticles for nanocomposites: A review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Dufresne, A. Polymer Nanocomposites from Biological Sources. Encycl. Nanosci. Nanotechnol. 2007, 21, 59–83. [Google Scholar]
- Jackson, D.S. STARCH | Structure, Properties, and Determination. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5561–5567. [Google Scholar] [CrossRef]
- Dai, L.; Li, C.; Zhang, J.; Cheng, F. Preparation and characterization of starch nanocrystals combining ball milling with acid hydrolysis. Carbohydr. Polym. 2018, 180, 122–127. [Google Scholar] [CrossRef]
- Putaux, J.-L.; Molina-Boisseau, S.; Momaur, T.; Dufresne, A. Platelet Nanocrystals Resulting from the Disruption of Waxy Maize Starch Granules by Acid Hydrolysis. Biomacromolecules 2003, 4, 1198–1202. [Google Scholar] [CrossRef]
- Wei, B.; Xu, X.; Jin, Z.; Tian, Y. Surface chemical compositions and dispersity of starch nanocrystals formed by sulfuric and hydrochloric acid hydrolysis. PLoS ONE 2014, 9, e86024. [Google Scholar] [CrossRef]
- Visakh, P.M. Chapter 1 Starch: State-of-the-Art, New Challenges and Opportunities. In Starch-Based Blends, Composites and Nanocomposites; The Royal Society of Chemistry: London, UK, 2016; pp. 1–16. [Google Scholar]
- Xu, Y.; Sismour, E.N.; Grizzard, C.; Thomas, M.; Pestov, D.; Huba, Z.; Wang, T.; Bhardwaj, H.L. Morphological, Structural, and Thermal Properties of Starch Nanocrystals Affected by Different Botanic Origins. Cereal Chem. J. 2014, 91, 383–388. [Google Scholar] [CrossRef]
- Odeniyi, M.A.; Omoteso, O.A.; Adepoju, A.O.; Jaiyeoba, K.T. Starch nanoparticles in drug delivery: A review. Polim. W Med. 2018, 48, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Lee, D.W. Fabrication and characterization of microcapsules with polyamide–polyurea as hybrid shell. J. Mater. Sci. 2012, 47, 2040–2044. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Ma, S.; Zong, W.; Luo, N.; Lv, M.; Hu, Y.; Zhou, L.; Han, X. Fabrication of pH sensitive microcapsules using soft templates and their application to drug release. RSC Adv. 2015, 5, 51271–51277. [Google Scholar] [CrossRef]
- Li, J.; Stover, H.D. Pickering emulsion templated layer-by-layer assembly for making microcapsules. Langmuir ACS J. Surf. Colloids 2010, 26, 15554–15560. [Google Scholar] [CrossRef]
- Madivala, B.; Vandebril, S.; Fransaer, J.; Vermant, J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Oza, K.P.; Frank, S.G. Microcrystalline Cellulose Stabilized emulsions. J. Dispers. Sci. Technol. 1986, 7, 543–561. [Google Scholar] [CrossRef]
- Oza, K.; Frank, S. Multiple Emulsions Stabilized by Colloidal Microcrystalline Cellulose. J. Dispers. Sci. Technol. 1989, 10, 163–185. [Google Scholar] [CrossRef]
- Ougiya, H.; Watanabe, K.; Morinaga, Y.; Yoshinaga, F. Emulsion-stabilizing Effect of Bacterial Cellulose. Biosci. Biotechnol. Biochem. 1997, 61, 1541–1545. [Google Scholar] [CrossRef] [Green Version]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir ACS J. Surf. Colloids 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Andresen, M.; Stenius, P. Water-in-oil Emulsions Stabilized by Hydrophobized Microfibrillated Cellulose. J. Dispers. Sci. Technol. 2007, 28, 837–844. [Google Scholar] [CrossRef]
- Blaker, J.J.; Lee, K.-Y.; Li, X.; Menner, A.; Bismarck, A. Renewable nanocomposite polymer foams synthesized from Pickering emulsion templates. Green Chem. 2009, 11, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Sebe, G.; Ham-Pichavant, F.; Pecastaings, G. Dispersibility and emulsion-stabilizing effect of cellulose nanowhiskers esterified by vinyl acetate and vinyl cinnamate. Biomacromolecules 2013, 14, 2937–2944. [Google Scholar] [CrossRef]
- Salajková, M.; Berglund, L.A.; Zhou, Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012, 22, 19798. [Google Scholar] [CrossRef]
- Cunha, A.G.; Mougel, J.B.; Cathala, B.; Berglund, L.A.; Capron, I. Preparation of double Pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir ACS J. Surf. Colloids 2014, 30, 9327–9335. [Google Scholar] [CrossRef]
- Ben Ayed, E.; Cochereau, R.; Dechance, C.; Capron, I.; Nicolai, T.; Benyahia, L. Water-In-Water Emulsion Gels Stabilized by Cellulose Nanocrystals. Langmuir ACS J. Surf. Colloids 2018, 34, 6887–6893. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Bian, W.; Feng, L.; Wu, Z.; Wang, P.; Zeng, X.; Wu, T. Stabilizing oil-in-water emulsions with regenerated chitin nanofibers. Food Chem. 2015, 183, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Pickering emulsions stabilized by native starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 142–149. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Yang, C. Emulsion stabilized by starch nanocrystals. Starch Stärke 2012, 64, 497–502. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sun, P.; Yang, C. Starch nanocrystals as particle stabilisers of oil-in-water emulsions. J. Sci. Food Agric. 2014, 94, 1802–1807. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Liu, C.; Li, Y.; Lu, C.; Wang, P. Fabrication of starch-based nanospheres to stabilize pickering emulsion. Carbohydr. Polym. 2012, 88, 1358–1363. [Google Scholar] [CrossRef]
- Song, X.; Pei, Y.; Zhu, W.; Fu, D.; Ren, H. Particle-stabilizers modified from indica rice starches differing in amylose content. Food Chem. 2014, 153, 74–80. [Google Scholar] [CrossRef]
- Marefati, A.; Sjöö, M.; Timgren, A.; Dejmek, P.; Rayner, M. Fabrication of encapsulated oil powders from starch granule stabilized W/O/W Pickering emulsions by freeze-drying. Food Hydrocoll. 2015, 51, 261–271. [Google Scholar] [CrossRef]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.-Y.; Kim, J.-W.; Fernandez-Nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Sontti, S.G.; Atta, A. Numerical Insights on Controlled Droplet Formation in a Microfluidic Flow-Focusing Device. Ind. Eng. Chem. Res. 2019, 59, 3702–3716. [Google Scholar] [CrossRef]
- Tong, W.; Song, X.; Gao, C. Layer-by-layer assembly of microcapsules and their biomedical applications. Chem. Soc. Rev. 2012, 41, 6103–6124. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Eyley, S.; Schütz, C.; van Gorp, H.; Rosenfeldt, S.; Van den Mooter, G.; Thielemans, W. Thermodynamic Study of the Interaction of Bovine Serum Albumin and Amino Acids with Cellulose Nanocrystals. Langmuir ACS J. Surf. Colloids 2017, 33, 5473–5481. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, S.; Chen, P.; Larsson, P.A.; Thielemans, W.; Wohlert, J.; Svagan, A.J. Toward Improved Understanding of the Interactions between Poorly Soluble Drugs and Cellulose Nanofibers. Langmuir ACS J. Surf. Colloids 2018, 34, 5464–5473. [Google Scholar] [CrossRef] [PubMed]
- Cranston, E.D.; Gray, D.G. Polyelectrolyte Multilayer Films Containing Cellulose: A Review. In Model Cellulosic Surfaces; American Chemical Society: Washington, DC, USA, 2009; Volume 1019, pp. 95–114. [Google Scholar]
- Villares, A.; Moreau, C.; Capron, I.; Cathala, B. Chitin nanocrystal-xyloglucan multilayer thin films. Biomacromolecules 2014, 15, 188–194. [Google Scholar] [CrossRef]
- Qi, Z.D.; Saito, T.; Fan, Y.; Isogai, A. Multifunctional coating films by layer-by-layer deposition of cellulose and chitin nanofibrils. Biomacromolecules 2012, 13, 553–558. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles-A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable Nanocomposite Films Based on Sodium Alginate and Cellulose Nanofibrils. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemahieu, L.; Bras, J.; Tiquet, P.; Augier, S.; Dufresne, A. Extrusion of Nanocellulose-Reinforced Nanocomposites Using the Dispersed Nano-Objects Protective Encapsulation (DOPE) Process. Macromol. Mater. Eng. 2011, 296, 984–991. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Huq, T.; Riedl, B.; Bouchard, J.; Salmieri, S.; Lacroix, M. Microencapsulation of nisin in alginate-cellulose nanocrystal (CNC) microbeads for prolonged efficacy against Listeria monocytogenes. Cellulose 2014, 21, 4309–4321. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Jamshidian, M.; Salmieri, S.; Desjardins, N.; Sahraoui, A.; Lacroix, M. Effect of cellulose nanocrystals on thyme essential oil release from alginate beads: Study of antimicrobial activity against Listeria innocua and ground meat shelf life in combination with gamma irradiation. Cellulose 2019, 26, 5247–5265. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, W.; Wang, Y.; Ke, C.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 112–120. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.; Hui, L.; Shang, Z.; Yuan, S.; Dai, L.; Liu, P.; Liu, X.; Ni, Y. Lignin-containing cellulose nanocrystals/sodium alginate beads as highly effective adsorbents for cationic organic dyes. Int. J. Biol. Macromol. 2019, 139, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Gencer, A.; Schutz, C.; Van Rie, J.; Eyley, S.; Thielemans, W. Thermodynamic Study of Ion-Driven Aggregation of Cellulose Nanocrystals. Biomacromolecules 2019, 20, 3181–3190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-cellulose nanocrystal microencapsulation to improve encapsulation efficiency and stability of entrapped fruit anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.H.; Zwieniecki, M.A. Physical Limits to Leaf Size in Tall Trees. Phys. Rev. Lett. 2013, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo-Rodriguez, S.A.; Allemann, E.; Fessi, H.; Doelker, E. Polymeric Nanoparticles for Oral Delivery of Drugs and Vaccines: A Critical Evaluation of In Vivo Studies. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 419–464. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-layer assembled thin films and microcapsules of nanocrystalline cellulose for hydrophobic drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef]
- Ye, C.; Malak, S.T.; Hu, K.; Wu, W.; Tsukruk, V.V. Cellulose Nanocrystal Microcapsules as Tunable Cages for Nano- and Microparticles. ACS Nano 2015, 9, 10887–10895. [Google Scholar] [CrossRef]
- Paulraj, T.; Riazanova, A.V.; Yao, K.; Andersson, R.L.; Mullertz, A.; Svagan, A.J. Bioinspired Layer-by-Layer Microcapsules Based on Cellulose Nanofibers with Switchable Permeability. Biomacromolecules 2017, 18, 1401–1410. [Google Scholar] [CrossRef]
- Paulraj, T.; Riazanova, A.V.; Svagan, A.J. Bioinspired capsules based on nanocellulose, xyloglucan and pectin—The influence of capsule wall composition on permeability properties. Acta Biomater. 2018, 69, 196–205. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Wieland, D.C.F.; Riazanova, A.V.; Dedinaite, A.; Gunther Pomorski, T.; Cardenas, M.; Svagan, A.J. Primary cell wall inspired micro containers as a step towards a synthetic plant cell. Nat. Commun. 2020, 11, 958. [Google Scholar] [CrossRef] [Green Version]
- Zykwinska, A.; Thibault, J.-F.; Ralet, M.-C. Competitive binding of pectin and xyloglucan with primary cell wall cellulose. Carbohydr. Polym. 2008, 74, 957–961. [Google Scholar] [CrossRef]
- Paulraj, T.; Wennmalm, S.; Riazanova, A.V.; Wu, Q.; Crespo, G.A.; Svagan, A.J. Porous Cellulose Nanofiber-Based Microcapsules for Biomolecular Sensing. ACS Appl. Mater. Interfaces 2018, 10, 41146–41154. [Google Scholar] [CrossRef] [PubMed]
- Mele, S.; Soderman, O.; Ljusberg-Wahren, H.; Thuresson, K.; Monduzzi, M.; Nylander, T. Phase behavior in the biologically important oleic acid/sodium oleate/water system. Chem. Phys. Lipids 2018, 211, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Mukhopadhyay, S.; Rokhlenko, Y.; Nejati, S.; Boltyanskiy, R.; Choo, Y.; Loewenberga, M.; Osuji, C.O. Highly stiff yet elastic microcapsules incorporating cellulose nanofibrils. Soft Matter 2017, 13, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Babayekhorasani, F.; Spicer, P.T. Soft Bacterial Cellulose Microcapsules with Adaptable Shapes. Biomacromolecules 2019, 20, 4437–4446. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Chen, P.; Rojas, R.; Hajian, A.; Berglund, L. Cellulose nanofibers enable paraffin encapsulation and the formation of stable thermal regulation nanocomposites. Nano Energy 2017, 34, 541–548. [Google Scholar] [CrossRef]
- Levin, D.; Saem, S.; Osorio, D.A.; Cerf, A.; Cranston, E.D.; Moran-Mirabal, J.M. Green Templating of Ultraporous Cross-Linked Cellulose Nanocrystal Microparticles. Chem. Mater. 2018, 30, 8040–8051. [Google Scholar] [CrossRef]
- Jativa, F.; Schütz, C.; Bergström, L.; Zhang, X.; Wicklein, B. Confined self-assembly of cellulose nanocrystals in a shrinking droplet. Soft Matter 2015, 11, 5374–5380. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.M.; Frka-Petesic, B.; Guidetti, G.; Kamita, G.; Consani, G.; Abell, C.; Vignolini, S. Hierarchical Self-Assembly of Cellulose Nanocrystals in a Confined Geometry. ACS Nano 2016, 10, 8443–8449. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Modulation of starch nanoparticle surface characteristics for the facile construction of recyclable Pickering interfacial enzymatic catalysis. Green Chem. 2019, 21, 2412–2427. [Google Scholar] [CrossRef]
- Jimenez-Saelices, C.; Trongsatitkul, T.; Lourdin, D.; Capron, I. Chitin Pickering Emulsion for Oil Inclusion in Composite Films. Carbohydr. Polym. 2020, 242, 116366. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Jimenez Saelices, C.; Capron, I. Design of Pickering Micro- and Nanoemulsions Based on the Structural Characteristics of Nanocelluloses. Biomacromolecules 2018, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Kuang, Y.; Guo, S.; Mo, L.; Ni, Y. Stabilization of Pickering emulsions with cellulose nanofibers derived from oil palm fruit bunch. Cellulose 2019, 27, 839–851. [Google Scholar] [CrossRef]
- Saidane, D.; Perrin, E.; Cherhal, F.; Guellec, F.; Capron, I. Some modification of cellulose nanocrystals for functional Pickering emulsions. Philos. Trans. Ser. AMath. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef]
- Nypelo, T.; Rodriguez-Abreu, C.; Kolen’ko, Y.V.; Rivas, J.; Rojas, O.J. Microbeads and hollow microcapsules obtained by self-assembly of pickering magneto-responsive cellulose nanocrystals. ACS Appl. Mater. Interfaces 2014, 6, 16851–16858. [Google Scholar] [CrossRef]
- Du, W.; Guo, J.; Li, H.; Gao, Y. Heterogeneously Modified Cellulose Nanocrystals-Stabilized Pickering Emulsion: Preparation and Their Template Application for the Creation of PS Microspheres with Amino-Rich Surfaces. ACS Sustain. Chem. Eng. 2017, 5, 7514–7523. [Google Scholar] [CrossRef]
- Werner, A.; Schmitt, V.; Sèbe, G.; Héroguez, V. Synthesis of surfactant-free micro- and nanolatexes from Pickering emulsions stabilized by acetylated cellulose nanocrystals. Polym. Chem. 2017, 8, 6064–6072. [Google Scholar] [CrossRef]
- Zhang, Y.; Karimkhani, V.; Makowski, B.T.; Samaranayake, G.; Rowan, S.J. Nanoemulsions and Nanolatexes Stabilized by Hydrophobically Functionalized Cellulose Nanocrystals. Macromolecules 2017, 50, 6032–6042. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Dubé, M.A.; Cranston, E.D. Cellulose Nanocrystals and Methyl Cellulose as Costabilizers for Nanocomposite Latexes with Double Morphology. ACS Sustain. Chem. Eng. 2017, 5, 10509–10517. [Google Scholar] [CrossRef]
- Jiménez Saelices, C.; Save, M.; Capron, I. Synthesis of latex stabilized by unmodified cellulose nanocrystals: The effect of monomers on particle size. Polym. Chem. 2019, 10, 727–737. [Google Scholar] [CrossRef]
- Butler, L.N.; Fellows, C.M.; Gilbert, R.G. Effect of surfactants used for binder synthesis on the properties of latex paints. Prog. Org. Coat. 2005, 53, 112–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, M.; Gabriel, M.S.; Teixeira Neto, A.A.; da Silva Bernardes, J.; Berry, R.; Tam, K.C. Polymeric hollow microcapsules (PHM) via cellulose nanocrystal stabilized Pickering emulsion polymerization. J. Colloid Interface Sci. 2019, 555, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, Y.; Pun, J.; Mohamed Osman, A.S.; Tam, K.C. Polydopamine microcapsules from cellulose nanocrystal stabilized Pickering emulsions for essential oil and pesticide encapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 403–413. [Google Scholar] [CrossRef]
- Zhang, Z.; Tam, K.C.; Wang, X.; Sèbe, G. Inverse Pickering Emulsions Stabilized by Cinnamate Modified Cellulose Nanocrystals as Templates to Prepare Silica Colloidosomes. ACS Sustain. Chem. Eng. 2018, 6, 2583–2590. [Google Scholar] [CrossRef]
- Svagan, A.J.; Musyanovych, A.; Kappl, M.; Bernhardt, M.; Glasser, G.; Wohnhaas, C.; Berglund, L.A.; Risbo, J.; Landfester, K. Cellulose nanofiber/nanocrystal reinforced capsules: A fast and facile approach toward assembly of liquid-core capsules with high mechanical stability. Biomacromolecules 2014, 15, 1852–1859. [Google Scholar] [CrossRef]
- Svagan, A.J.; Bender Koch, C.; Hedenqvist, M.S.; Nilsson, F.; Glasser, G.; Baluschev, S.; Andersen, M.L. Liquid-core nanocellulose-shell capsules with tunable oxygen permeability. Carbohydr. Polym. 2016, 136, 292–299. [Google Scholar] [CrossRef]
- Nordenstrom, M.; Riazanova, A.V.; Jarn, M.; Paulraj, T.; Turner, C.; Strom, V.; Olsson, R.T.; Svagan, A.J. Superamphiphobic coatings based on liquid-core microcapsules with engineered capsule walls and functionality. Sci. Rep. 2018, 8, 3647. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. Fabrication of melamine–urea–formaldehyde/paraffin microcapsules modified with cellulose nanocrystals via in situ polymerization. J. Mater. Sci. 2019, 54, 7383–7396. [Google Scholar] [CrossRef]
- Perrin, E.; Bizot, H.; Cathala, B.; Capron, I. Chitin nanocrystals for Pickering high internal phase emulsions. Biomacromolecules 2014, 15, 3766–3771. [Google Scholar] [CrossRef]
- Haaj, S.B.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystal stabilized Pickering emulsion polymerization for nanocomposites with improved performance. ACS Appl. Mater. Interfaces 2014, 6, 8263–8273. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, C.; Zou, S.; Liu, H.; Tong, Z. Chitosan nanoparticles as particular emulsifier for preparation of novel pH-responsive Pickering emulsions and PLGA microcapsules. Polymer 2012, 53, 1229–1235. [Google Scholar] [CrossRef]






| Nanomaterial | Encapsulated Specie | Properties Studied | Ref |
|---|---|---|---|
| CNC ChiNC SNC | Theophilline | Swelling in water - loading and release | [117] |
| CNC | Probiotics | swelling and dissolution under simulated gastrointestinal conditions | [122] |
| CNC | Nisin | loading and release | [123] |
| CNC | Thyme oil | loading and release | [124] |
| Bacterial CNC | α-Calcidol | loading and release | [125] |
| CNC | Methylene blue | Thermodynamics of interactions | [126] |
| CNC (containing lignin) | Methylene blue | Thermodynamics of interactions | [128] |
| CNC | Pb2+ | Thermodynamics of interactions | [127] |
| Template Used | Coating Components | Encapsulated Species | Properties Studied | Size (µm) | Ref |
|---|---|---|---|---|---|
| Melamine formaldeyde | (chitosan /CNC)n | Doxorubicin hydrochloride | Loading and release | 3.3–3.5 | [133] |
| SiO2 | PEI(CNC)n | Polystyrene beads | Permeability in water | 3.8 ± 0.5 | [134] |
| CaCO3 | CNF/AP/ XyG (AP/CNF)5AP/CNF | Dextran | Permeability in water | 16 ± 4 | [135] |
| CaCO3 | (CNF-XyG)5 (CNF/XyG/CNF/AP)2/CNF/XG | Dextran BSA | Permeability in water/NaCl and cell growth media | 16 ± 4 | [136] |
| CaCO3 | (CNF/XyG/CNF/AP)2CNF | Dextran | Permeability in biological buffer - GOx loading efficiency – enzyme activity | 12 ± 2 | [139] |
| Oil-in-water emulsion | CNF/Pectin | Porosity – pH dependent structure and expansion | 27 | [137] | |
| Water-in-oil emulsion | CNC/cationic polymer | Mechanical properties | 303 ± 3.4 | [141] | |
| Oil-in-water emulsion | Bacterial cellulose | Porosity, mechanical properties | from 100 to few cm | [142] | |
| Oil-in-water emulsion | CNF | Paraffin | Mechanical and thermal properties | 5–10 | [143] |
| Oil-in-water emulsion | ChiNC | Paraffin | 2–5 | [148] | |
| Water-in-oil | CNC(SO4) CNC(aldehyde)-CNC (hydrazone) | Swelling, porosity, self-assembly | > 300 | [144] | |
| Water-in- toluene/ethanol | CNC(COOH) | Self-assembly | 300–800 | [145] | |
| Water-in-hexadecane | CNC(SO4) | Self-assembly | ≈ 20 | [146] | |
| n-Heptane-in-water | SNC | enzyme | Catalytic activity | 5–30 | [147] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, S.; Villares, A. Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules 2020, 25, 4420. https://doi.org/10.3390/molecules25194420
Lombardo S, Villares A. Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules. 2020; 25(19):4420. https://doi.org/10.3390/molecules25194420
Chicago/Turabian StyleLombardo, Salvatore, and Ana Villares. 2020. "Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials" Molecules 25, no. 19: 4420. https://doi.org/10.3390/molecules25194420
APA StyleLombardo, S., & Villares, A. (2020). Engineered Multilayer Microcapsules Based on Polysaccharides Nanomaterials. Molecules, 25(19), 4420. https://doi.org/10.3390/molecules25194420