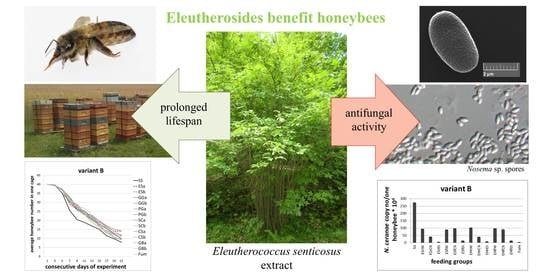
Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis
Abstract
:1. Introduction
2. Results
2.1. Results of Cage Tests
- A.
- Honeybees in variant “A” (control, impact of extracts on uninfected honeybees) served as a control and were not infected with N. ceranae. Feeding group SS (sucrose syrup) served as a negative control with uninfected honeybees fed solely with 1:1 w:v [1:1 weight:volume sugar:water] sucrose solution and not treated with extracts.
- B.
- In variant “B” (impact of extracts on the treatment of nosemosis), to check whether the supplementation of honeybee diets with extracts influences the course of nosemosis, firstly, honeybees were Nosema ceranae-infected, and subsequently fed with a sucrose solution, containing extracts. Feeding group SS served as a negative control with Nosema ceranae-infected honeybees fed solely with a 1:1 w:v sucrose solution, not treated with extracts, and Fum (fumagillin), served as a positive control in the treatment of nosemosis.
- C.
- In variant “C” (impact of extracts on the prevention of nosemosis), to check whether the supplementation of honeybee diets with extracts protects honeybees against nosemosis, firstly, honeybees were fed with a sucrose solution supplemented with extracts, and after that, were infected with N. ceranae spores. Feeding group SS served as a negative control with Nosema ceranae-infected honeybees fed solely with a 1:1 w:v sucrose solution and not treated with extracts.
2.1.1. Results of Cage Tests: Screening of Commercial Plant Extracts
2.1.2. Results of Cage Tests: Screening for the Best Method to Obtain the Eleutherococcus Extract
2.1.3. Results of Cage Tests: Screening for the Best Eleutherococcus Dose
2.2. Additional Tests
2.2.1. Results of Field Tests
2.2.2. Results of LIVE/DEAD Tests and Nosema Spore Cell Wall Analysis under Scanning Electron Microscopy (SEM)
2.3. Phytochemical Analysis of the Extracts and Honey
3. Discussion
4. Methods
4.1. Standards and Reagents
4.2. Plant Material
4.3. Dried Material Extraction with 75% Ethanol
4.4. Dried Material Extraction with Chloroform
4.5. Infusion Preparation
4.6. Phytochemical Analysis of the Extracts and Honey
4.7. Phytochemical Analysis of the Extracts: Stability of the Extracts in Time—HPLC-DAD Conditions of Eleutheroside B and E and Naringenin
4.8. Phytochemical Analysis of the Honey: Eleutherococcus Extract Residues in Honey—HPLC-DAD of Eleutheroside B and E and Naringenin
4.9. Animals, Cage and Field Tests
4.10. Cage Tests: The Scheme of Administered Experiments
4.10.1. Cage Tests: Screening of Commercial Plant Extracts
4.10.2. Cage Tests: Screening for the Best Method to Obtain the Eleutherococcus Extract
4.10.3. Cage Tests: Screening for the Best Eleutherococcus Dose
4.11. Isolation of Total DNA from Honeybees and Molecular Detection of N. ceranae
4.12. Estimation of the Level of Nosemosis Level
4.13. Additional Tests
4.13.1. Field Tests
4.13.2. Impact of the Pre-Treatment of Nosema ceranae Spores with Eleutherococcus Extracts on the Spores’ Viability
4.13.3. Nosema Spores’ Viability Control
4.13.4. The Nosema Spore Cell Wall Analysis Under Scanning Electron Microscopy (SEM)
4.14. Statistical Analyses
5. Conclusions and a Future Perspective
6. Highlights
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslan, C.E.; Liang, C.T.; Galindo, B.; Kimberly, H.; Topete, W. The role of honey bees as pollinators in natural areas. Nat. Areas J. 2016, 36, 478–488. [Google Scholar] [CrossRef]
- Prasad, P.Y.; Mackereth, R.W.; Hanley, R.S.; Qin, W. Honey Bees (Apis mellifera L.) and Pollination Issues: Current status, impacts and potential drivers of decline. J. Agric. Sci. 2015, 7, 93. [Google Scholar]
- Zalasiewicz, J.; Waters, C.N.; Summerhayes, C.P.; Wolfe, A.P.; Barnosky, A.D.; Cearreta, A.; Crutzen, P.; Ellis, E.; Fairchild, I.J.; Gałuszka, A.; et al. The Working Group on the Anthropocene: Summary of evidence and interim recommendations. Anthropocene 2017, 19, 55–60. [Google Scholar] [CrossRef]
- Crutzen, P.J. Geology of mankind. Nature 2002, 415, 23. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Stoermer, E.F. The Anthropocene. Global Change Newsletter. Int. Geosph. Biosph. Programme 2000, 41, 17–18. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R. The misunderstood sixth mass extinction. Science 2018, 360, 1080–1081. [Google Scholar]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef]
- Fries, I.; Martín, R.; Meana, A.; García–Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Chemurot, M.; Smet, L.; Brunain, M.; De Rycke, R.; de Graaf, D.C. Nosema neumanni n. sp. (Microsporidia, Nosematidae), a new microsporidian parasite of honeybees, Apis mellifera in Uganda. Eur. J. Protistol. 2017, 61, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Panek, J.; Paris, L.; Roriz, D.; Mone, A.; Dubuffet, A.; Delbac, F.; Diogon, M.; El Alaoui, H. Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis Mellifera. J. Invertebr. Pathol. 2018, 159, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Demetraki-Paleolog, J. Differentiation of Nosema apis and Nosema ceranae spores under Scanning Electron Microscopy (SEM). J. Apicult. Res. 2014, 53, 537–544. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Gancarz, M.; Hurd, P.J.; Borsuk, G.; Wiącek, D.; Nawrocka, A.; Strachecka, A.; Załuski, D.; Paleolog, J. Changes in the bioelement content of summer and winter western honeybees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 2018, 13, e0200410. [Google Scholar] [CrossRef]
- Huang, Q.; Evans, J.D. Targeting the honey bee gut parasite Nosema ceranae with siRNA positively affects gut bacteria. BMC Microbiol. 2020, 20, 258. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Paleolog, J.; Borsuk, G. Nosema ceranae Infection Promotes Proliferation of Yeasts in Honey Bee Intestines. PLoS ONE 2016, 11, e0164477. [Google Scholar] [CrossRef] [Green Version]
- Copley, T.R.; Jabaji, S.H. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. J. Appl. Microbiol. 2012, 112, 15–24. [Google Scholar] [CrossRef]
- Wang, D.I.; Moeller, F.E. Ultrastructural changes in the hypopharyngeal gland of worker honey bees infected by Nosema apis. J. Invert. Pathol. 1971, 17, 308–320. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Anusiewicz, M.; Mułenko, W. Location of Nosema spp. spores within body of honey bee. Med. Weter. 2012, 68, 618–621. [Google Scholar]
- Lecocq, A.; Jensen, A.B.; Kryger, P.; Nieh, J.C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 2016, 6, 22042. [Google Scholar] [CrossRef] [PubMed]
- Tofilski, A.; Kopel, J. The influence of Nosema apis on maturation and flight activity of honey bee drones. Pszczel. Zesz. Nauk. 1996, 40, 55–60. [Google Scholar]
- Peng, Y.; Baer–Imhoof, B.; Millar, A.H.; Baer, B. Consequences of Nosema apis infection for male honeybees and their fertility. Sci. Rep. 2015, 5, 10565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2001, 91, 409–420. [Google Scholar] [CrossRef]
- Burnham, A.J. Scientific Advances in Controlling Nosema ceranae (Microsporidia) Infections in Honey Bees (Apis mellifera). Front. Vet. Sci. 2019, 6, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, S.; Xu, Y.; Gong, H.; Wu, Y.; Chen, Y.; Zheng, H. Protective potential of Chinese herbal extracts against microsporidian Nosema ceranae, an emergent pathogen of western honeybees, Apis mellifera L. J. Asia Pac. Entomol. 2019. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.K.; Lee, J.K. Evaluation of antimicrosporidian activity of plant extracts on Nosema ceranae. J. Apic. Sci. 2016, 60, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, J.J.; Leslie, J.; Tarpy, D.; Palmer-Young, E.; Irwin, R.; Adler, L.S. Medicinal value of sunflower pollen against bee pathogens. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; MartínHernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Wilk, J. Impact of vertebrate probiotics on honeybee yeast microbiota and on the course of nosemosis. Med. Weter. 2016. [Google Scholar] [CrossRef]
- Dumitru, A.; Chioveanu, G.; Ionita, M.; Dobre, G.; Mitrea, I.L. “In vitro” studies on using natural essential oils in treatment of nosemosis in honeybees: Determination of the therapeutic Dose. Sci. Work. Vet. Medic. Ser. C 2017, 63, 165–170. [Google Scholar]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis Consumption reduces Nosema ceranae infection of European honeybees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Palmer-Young, E.C.; Tozkar, C.Ö.; Schwarz, R.S.; Chen, Y.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Nectar and Pollen Phytochemicals Stimulate Honey Bee (Hymenoptera: Apidae) Immunity to Viral Infection. J. Econ. Entomol. 2017, 110, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.H.S.A. Dietary Phytochemicals, Honey Bee Longevity and Pathogen Tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, M.P.; Garrido, P.M.; Gende, L.B.; Rossini, C.; Hermida, L.; Marcángeli, J.A.; Eguaras, M.J. Oral administration of essential oils and main components: Study on honeybee survival and Nosema ceranae development. J. Apic. Res. 2017, 56, 616–624. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae Escapes Fumagillin Control in Honey Bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef]
- van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef]
- Dimpfel, W.; Schombert, L.; Keplinger–Dimpfel, I.K.; Panossian, A. Effects of an adaptogenic extract on electrial activity of the brain in elderly subjects with mild cognitive impairment: A randomized, double–blind, placebo–controlled, two–armed cross–over study. Pharmaceuticals 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieśla, Ł.; Waksmundzka-Hajnos, M.; Załuski, D.; Smolarz, H.; Hajnos, M. HPTLC–densitometric method for determination of eleutherosides B, E and E1 in different Eleutherococcus species. J. Chromatogr. Sci. 2011, 49, 182–188. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Choromańska, A.; Abel, R.; Preissner, R.; Saczko, J.; Matkowski, A.; Hadzik, J. Cytotoxic Effect of Vanicosides A and B from Reynoutria sachalinensis against Melanotic and Amelanotic Melanoma Cell Lines and in silico Evaluation for Inhibition of BRAFV600E and MEK1. Int. J. Mol. Sci. 2020, 21, 4611. [Google Scholar] [CrossRef] [PubMed]
- Bączek, K. Accumulation of biologically active compounds in Eleuthero (Eleutherococcus senticosus /Rupr. et Maxim./ Maxim.) grown in Poland. Herba Pol. 2009, 55, 7–13. [Google Scholar]
- Bączek, K.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z. Accumulation of phenolics in eleuthero (Eleutherococcus senticosus (Rupr. t Maxim.) Maxim.) as affected by plant development. Acta Sci. Pol. Technol. Cultus. 2017, 16, 89–99. [Google Scholar]
- Bączek, K. Diversity of Eleutherococcus genus in respect of biologically active compounds accumulation. Herba Pol. 2014, 60, 1–10. [Google Scholar]
- Ahn, J.; Um, M.Y.; Lee, H.; Jung, C.H.; Heo, S.H.; Ha, T.Y. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid. Complement. Altern. Med. 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Załuski, D.; Olech, M.; Kuźniewski, R.; Verpoorte, R.; Nowak, R.; Smolarz, H.D. LC–ESI–MS/MS profiling of phenolics from Eleutherococcus spp. inflorescences, structure–activity relationship as antioxidants, inhibitors of hyaluronidase and acetylcholinesterase. Saudi Pharm. J. 2017, 25, 734–743. [Google Scholar]
- Venkateswara, R.P.; Kiran, S.D.V.S.; Rohini, P.; Bhagyasree, P. Flavonoid: A review on Naringenin. J. Pharmacog. Phytochemistr. 2017, 6, 2778–2783. [Google Scholar]
- Borges, D.; Guzman-Novoa, E. Goodwin PH (2020) Control of the microsporidian parasite Nosema ceranae in honeybees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef]
- Grandjean, P. Paracelsus Revisited: The Dose Concept in a Complex World. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.P.; Fernández, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Pohorec, K. Laboratory studies on the effect of standardized Artemisia absinthium L. extract on Nosema apis infection in the worker Apis mellifera. J. Apis. Sci. 2004, 48, 131–136. [Google Scholar]
- Damiani, N.; Fernández, N.J.; Porrini, M.P.; Gende, L.B.; Álvarez, E.; Buffa, F.; Brasesco, C.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J. Laurel leaf extracts for honeybee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Janeczko, Z. Variation in phytochemicals and bioactivity of the fruits of Eleutherococcus species cultivated in Poland. Nat. Prod. Resear. 2015, 29, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín–Hernández, R.; Meana, A.; Higes, M. Screening alternative therapies to control Nosemosis type C in honey bee (Apis mellifera iberiensis) colonies. Res. Vet. Sci. 2013, 95, 1041–1045. [Google Scholar] [CrossRef]
- Tauber, J.P.; Collins, W.R.; Schwarz, R.S.; Chen, Y.; Grubbs, K.; Huang, Q.; Lopez, D.; Peterson, R.; Evans, J.D. Natural Product Medicines for Honey Bees: Perspective and Protocols. Insects 2019, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Ptaszyńska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka–Jasińska, K.; Gryko, D. Porphyrins inactivate Nosema spp. microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Revenis, C. Role and Importance of Phenoloxidase in Insect Hemostasis. J. Innate Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef]
- Ferrandon, D.; Imler, J.L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Cytryńska, M.; Mułenko, W.; Zdybicka–Barabas, A.; Borsuk, G.; Załuski, D. Plant extracts for use in the treatment of honey bees nosemosis and to enhance bee resistance. PL. 232685, 19 July 2019. [Google Scholar]
- Ptaszyńska, A.A.; Borsuk, G.; Zdybicka–Barabas, A.; Cytryńska, M.; Małek, W. Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasit. Res. 2016, 115, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrejko, M.; Zdybicka–Barabas, A.; Cytryńska, M. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J. Invertebr. Pathol. 2014, 115, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Zdybicka–Barabas, A.; Mak, P.; Jakubowicz, T.; Cytryńska, M. Lysozyme and defense peptides as suppressors of phenoloxidase activity in Galleria mellonella. Arch. Insect Biochem. Physiol. 2014, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stączek, S.; Zdybicka–Barabas, A.; Pleszczyńska, M.; Wiater, A.; Cytryńska, M. Aspergillus niger α–1,3–glucan acts as a virulence factor by inhibiting the insect phenoloxidase system. J. Invertebr. Pathol. 2020, 171, 107341. [Google Scholar] [CrossRef] [PubMed]
- Wojda, I.; Cytryńska, M.; Zdybicka–Barabas, A.; Kordaczuk, J. Insect Defense Proteins and Peptides. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins; Hoeger, U., Harris, J., Eds.; Subcellular Biochemistry: Cham, Switzerland, 2020; Volume 94. [Google Scholar] [CrossRef]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmac. 2000, 72, 345–393. [Google Scholar] [CrossRef]
- Asea, A.; Kaur, P.; Panossian, A.; Wikman, K.G. Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts. Phytomedicine 2013, 20, 1323–1329. [Google Scholar] [CrossRef]
- Traver, B.E.; Williams, M.R.; Fell, R.D. Comparison of within hive sampling and seasonal activity of Nosema ceranae in honey bee colonies. J. Invertebr. Pathol. 2012, 109, 187–193. [Google Scholar] [CrossRef]
- Mulholland, G.E.; Traver, B.E.; Johnson, N.G.; Fell, R.D. Individual variability of Nosema ceranae infections in Apis mellifera colonies. Insects 2012, 3, 1143–1155. [Google Scholar] [CrossRef] [Green Version]
- Gisder, S.; Hedtke, K.; Möckel, N.; Frielitz, M.-C.; Linde, A.; Genersch, E. Five–year cohort study of Nosema spp. in Germany: Does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microbiol. 2010, 76, 3032–3038. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, K.; Olech, M.; Abramek, J.; Pietrzak, W.; Kuźniewski, R.; Bogucka-Kocka, A.; Nowak, R.; Ptaszyńska, A.A.; Rapacka-Gackowska, A.; Skalski, T.; et al. Eleutherococcus Species Cultivated in Europe: A New Source of Compounds with Antiacetylcholinesterase, Antihyaluronidase, Anti-DPPH, and Cytotoxic Activities. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.G.; Baker, I. Studies of nectar-constitution and pollinator–plant coevolution. In Coevolution of plants and animals; Gilbert, L.E., Raven, P.H., Eds.; The University of Texas Press: Austin, TX, USA; Springer: Boston, MA, USA, 1975; pp. 100–140. [Google Scholar]
- Rhoades, D.F.; Bergdahl, J.C. Adaptive significance of toxic nectar. Am. Nat. 1981, 117, 798–803. [Google Scholar] [CrossRef]
- Tumiłowicz, J.; Banaszczak, P. Trees and shrubs of Aquifoliaceae family in Rogów and Glinna arboreta. Rocz. Dendrol. 2007, 55, 41–56. [Google Scholar]
- eFloras. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA. Available online: http://www.efloras.org (accessed on 1 October 2015).
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; Mcmahon, D.P.; Martínhernández, R.; Natsopoulou, M.; et al. Standard methods for nosema research. In The COLOSS BEEBOOK: Volume II: Standard Methods for Apis Mellifera Pest and Pathogen Research; Dietemann, V., Ellis, J.D., Neumann, P., Eds.; Taylor and Francis Ltd.: Milton Park, UK, 2013; Volume 51. [Google Scholar] [CrossRef] [Green Version]
- Martín–Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantwell, G.E. Standard methods for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Hornitzky, M. Nosema Disease—Literature review and three surveys of beekeepers—Part 2; Rural Industries Research and Development Corporation: New South Wales, Australia, 2008. [Google Scholar]
- Bourgeois, A.L.; Rinderer, T.E.; Beaman, L.D.; Danka, R.G. Genetic detection and quantification of Nosema apis and N. ceranae in the honey bee. J. Invertebr. Pathol. 2010, 103, 53–58. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.; Chen, Y.; Budge, G.; Cornman, R.; Rúa, P.D.; Miranda, J.D.; Forêt, S.; Foster, L.; Gauthier, L.; et al. Standard methodologies for molecular research in Apis mellifera. In The COLOSS BEEBOOK, Volume I: Standard Methods for Apis Mellifera Research V; Dietemann, J.D., Ellis, P.N., Eds.; Taylor and Francis Ltd.: Milton Park, UK, 2013; Volume 52. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Evans, J.; Zhou, L.; Boncristiani, H.; Kimura, K.; Xiao, T.; Litkowski, A.M.; Pettis, J. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invertebr. Pathol. 2009, 101, 204–209. [Google Scholar] [CrossRef]
- Chen, Y.P.; Higgins, J.A.; Feldlaufer, M.F. Quantitative real–time reverse transcription PCR analysis of deformed Wing virus infection in the honeybee (Apis mellifera L.). Appl. Environ. Microbiol. 2005, 71, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Meana, A.; Martín–Hernández, R.; Higes, M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J. Apic. Res. Bee World 2010, 49, 212–214. [Google Scholar] [CrossRef]
- Peng, Y.; Lee–Pullen, T.F.; Heel, K.; Millar, A.H.; Baer, B. Quantifying spore viability of the honey bee pathogen Nosema apis using flow cytometry. Cytom. Part A 2014, 85, 454–462. [Google Scholar] [CrossRef]
Sample Availability: Samples of honeybees and extracts are available from the authors. |






| Honeybee Longevity | Nosema Infection | Food Intake | |
|---|---|---|---|
| Eleutherococcus senticosus (ES) | ++ | ++ | + |
| Garcinia gummi-gutta (GG) | + | + | + |
| Panax ginseng (PG) | - | + | - |
| Schisandra chinensis (SC) | + | + | + |
| Camellia sinensis (CS) | - | - | - |
| Ginkgo biloba (GB) | + | - | + |
| Fumagillin (Fum) | - | ++ | + |
| The Type of Extraction Plant Species | Water | Chloroform | 75% Ethanol |
|---|---|---|---|
| E. senticosus root | ESrW | ESrCh | ESrEt |
| E. senticosus fruit | ESfW | ESfCh | ESfEt |
| E. henryi root | EHrW | EHrCh | EHrEt |
| E. henryi fruit | EHfW | EHfCh | EHfEt |
| Section no. | Test | Feeding Groups | No. of cages in Each Feeding Group | No. of Bees in Each Cage | No. of Repeats | Total Honeybee Number in Each Experiment in One Treatment A, B or C |
|---|---|---|---|---|---|---|
| Section 4.10.1 | Screening for commercial plant extracts. | 14 | 3 | 40 | 2 | 3360 |
| Section 4.10.2 | Screening for the best method to obtain the Eleutherococcus extract | 14 | 3 | 40 | 2 | 3360 |
| Section 4.10.3 | Screening for the best Eleutherococcus extract dose | 7 | 6 | 40 | 2 | 3360 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptaszyńska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis. Molecules 2020, 25, 4452. https://doi.org/10.3390/molecules25194452
Ptaszyńska AA, Załuski D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis. Molecules. 2020; 25(19):4452. https://doi.org/10.3390/molecules25194452
Chicago/Turabian StylePtaszyńska, Aneta A., and Daniel Załuski. 2020. "Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis" Molecules 25, no. 19: 4452. https://doi.org/10.3390/molecules25194452
APA StylePtaszyńska, A. A., & Załuski, D. (2020). Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis. Molecules, 25(19), 4452. https://doi.org/10.3390/molecules25194452