Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome
Abstract
:1. Introduction
2. Chemical Properties
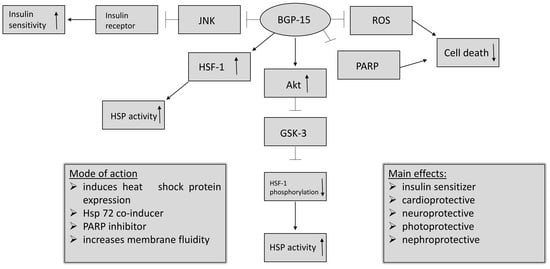
3. Mechanisms of Effects
- BGP-15 inhibits the acetylation of heat shock factor 1 (HSF-1), thus increasing heat shock protein (HSP) induction. It is a co-inducer of Hsp72 [3].
- BGP-15 is a poly (adenosine 5′-diphosphate)–ribose] polymerase 1 (PARP-1) inhibitor, and is able to reduce mitochondrial ROS production as well. Pharmacological inhibition of PARP and reducing the production of reactive oxygen species (ROS) can be effective in a wide selection of diseases, by protecting the cells against death [11].
4. Pharmacology
5. Preclinical Studies
5.1. Cell Culture Models
5.2. Animal Models
6. Potential Effects
6.1. Cardiovascular Effects
6.2. The Effect of BGP-15 in Duchenne Muscular Dystrophy
6.3. Chemo and Cytoprotective Effects of BGP-15
6.4. BGP-15’s Effect in Liver Injury
6.5. Insulin Sensitizing Effect of BGP-15
6.6. Effects of BGP-15 in Skin Injury, TBI, and VIDD
6.7. Gynecological Diseases and ROS-Related and Inflammatory Diseases
7. Aspects of Pharmaceutical Technology
8. Clinical Trials of BGP-15
9. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagy, G.; Szarka, A.; Lotz, G.; Dóczi, J.; Wunderlich, L.; Kiss, A.; Jemnitz, K.; Veres, Z.; Bánhegyi, G.; Schaff, Z.; et al. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 2010, 243, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Origo. Available online: https://www.origo.hu/egeszseg/20120419-tudomanyos-attorest-hozhat-a-magyar-felfedezes-cukorbetegseg-inzulin.html (accessed on 28 April 2019).
- Literáti-Nagy, B.; Kulcsár, E.; Literáti-Nagy, Z.; Buday, B.; Péterfai, É.; Horváth, T.; Tory, K.; Kolonics, A.; Fleming, A.; Mandl, J.; et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: A proof of concept randomized double-blind clinical trial. Horm. Metab. Res. 2009, 41, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Literáti-Nagy, Z.; Tory, K.; Literáti-Nagy, B.; Bajza, Á.; Vígh, L., Jr.; Vígh, L.; Szilvássy, Z. Synergetic insulin sensitizing effect of rimonabant and BGP-15 in zucker-obes rats. Pathol. Oncol. Res. 2013, 19, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Chemical Structure. Available online: https://www.selleckchem.com/products/bgp-15.html (accessed on 10 May 2019).
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/b4813?lang=hu®ion=HU (accessed on 10 June 2019).
- U.S. National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/bgp-15. (accessed on 18 April 2019).
- Hooper, P.L.; Balogh, G.; Rivas, E.; Kavanagh, K.; Vigh, L. The importance of the cellular stress response in the pathogenesis and treatment if type 2 diabetes. Cell Stress Chaperones. 2014, 19, 447–464. [Google Scholar] [CrossRef] [Green Version]
- Crul, T.; Toth, N.; Piotto, S.; Literati-Nagy, P.; Tory, K.; Haldimann, P.; Kalmar, B.; Greensmith, L.; Torok, Z.; Balogh, G.; et al. Hydroximic Acid Derivatives: Pleiotropic Hsp Co-Inducers Restoring Homeostasis and Robustness. Curr. Pharm. Des. 2013, 19, 309–346. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.; López, J.C.; Schubert, C.; Stevens, K. Melting away. Nat. Med. 2008, 8, 123. [Google Scholar]
- Szabados, E.; Literati-Nagy, P.; Farkas, B.; Sumegi, B. BGP-15, a nicotinic amidoxime derivate protecting heart from ischemia reperfusion injury through modulation of poly(ADP-ribose) polymerase. Biochem. Pharmacol. 2000, 59, 937–945. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef] [Green Version]
- Gungor, B.; Vanharanta, L.; Hölttä-Vuori, M.; Pirhonen, J.; Petersen, N.H.T.; Gramolelli, S.; Ojala, P.M.; Kirkegaard, T.; Ikonen, E. HSP70 induces liver X receptor pathway activation and cholesterol reduction in vitro and in vivo. Mol. Metab. 2019, 28, 135–143. [Google Scholar] [CrossRef]
- Literáti-Nagy, B.; Tory, K.; Peitl, B.; Bajza, Á.; Korányi, L.; Literáti-Nagy, Z.; Hooper, P.L.; Vígh, L.; Szilvássy, Z. Improvement of Insulin Sensitivity by a Novel Drug Candidate, BGP-15, in Different Animal Studies. Metab. Syndr. Relat. Disord. 2014, 12, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Gehrig, S.M.; van der Poel, C.; Sayer, T.A.; Schertzer, J.D.; Henstridge, D.C.; Church, J.E.; Lamon, S.; Russel, A.P.; Davies, K.E.; Febbraio, M.A.; et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature 2012, 484, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Halmosi, R.; Berente, Z.; Osz, E.; Toth, K.; Literati-Nagy, P.; Sumegi, B. Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system. Mol. Pharmacol. 2001, 59, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, M.; Priksz, D.; Gesztelyi, R.; Kiss, R.; Hollos, N.; Varga, B.; Nemeth, J.; Toth, A.; Papp, Z.; Szilvassy, Z.; et al. The Drug Candidate BGP-15 Delays the Onset of Diastolic Dysfunction in the Goto-Kakizaki Rat Model of Diabetic Cardiomyopathy. Molecules 2019, 24, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapra, G.; Tham, Y.K.; Cemerlang, N.; Matsumoto, A.; Kiriazis, H.; Bernardo, B.C.; Henstridge, D.C.; Ooi, J.Y.Y.; Pretorius, L.; Boey, E.Y.H.; et al. The small-molecule BGP-15 protects against heart failure and atrial fibrillation in mice. Nat. Commun. 2014, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bárdos, G.; Móricz, K.; Jaszlits, L.; Rabloczky, G.; Tory, K.; Rácz, I.; Bernáth, S.; Sümegi, B.; Farkas, B.; Literáti-Nagy, B.; et al. BGP-15, a hydroximic acid derivative, protects against cisplatin- or taxol-induced peripheral neuropathy in rats. Toxicol. Appl. Pharmacol. 2003, 190, 9–16. [Google Scholar] [CrossRef]
- Farkas, B.; Magyarlaki, M.; Csete, B.; Nemeth, J.; Rabloczky, G.; Bernath, S.; Literáti-Nagy, P.; Sümegi, B. Reduction of acute photodamage in skin by topical application of a novel PARP inhibitor. Biochem. Pharmacol. 2002, 63, 921–932. [Google Scholar] [CrossRef]
- Racz, I.; Tory, K.; Gallyas, F., Jr.; Berente, Z.; Osz, E.; Jaszlits, L.; Bernath, S.; Sumegi, B.; Rabloczky, G.; Literati-Nagy, P. BGP-15—A novel poly(ADP-ribose) polymerase inhibitor—protects against nephrotoxicity of cisplatin without compromising its antitumor activity. Biochem. Pharmacol. 2002, 63, 1099–1111. [Google Scholar] [CrossRef]
- Eroglu, B.; Kimbler, D.E.; Pang, J.; Choi, J.; Moskophidis, D.; Yanasak, N.; Dhandapani, K.M.; Mivechi, N.F. Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J. Neurochem. 2014, 130, 626–641. [Google Scholar] [CrossRef] [Green Version]
- Salah, H.; Li, M.; Cacciani, N.; Gastaldello, S.; Ogilvie, H.; Akkad, H.; Namuduri, A.V.; Morbidoni, V.; Artemenko, K.A.; Balogh, G.; et al. The chaperone co-inducer BGP-15 alleviates ventilation-induced diaphragm dysfunction. Sci. Transl. Med. 2016, 8, 350ra103. [Google Scholar] [CrossRef]
- Sumegi, K.; Fekete, K.; Antus, C.; Debreczeni, B.; Hocsak, E.; Gallyas, F., Jr.; Sumegi, B.; Szabo, A. BGP-15 protects against oxidative stress- or lipopolysaccharide-induced mitochondrial destabilization and reduces mitochondrial production of reactive oxygen species. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Polson, A.G.; Fuji, R.N. The successes and limitations of preclinical studies in predicting the pharmacodynamics and safety of cell-surface-targeted biological agents in patients. Br. J. Pharmacol. 2012, 166, 1600–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budzyński, M.A.; Crul, T.; Himanen, S.V.; Toth, N.; Otvos, F.; Sistonen, L.; Vigh, L. Chaperone co-inducer BGP-15 inhibits histone deacetylases and enhances the heat shock response through increased chromatin accessibility. Cell Stress Chaperones. 2017, 22, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batool, T.; Fang, J.; Jansson, V.; Zhao, H.; Gallant, C.; Moustakas, A.; Li, J. Upregulated BMP-Smad signaling activity in the glucuronyl C5-epimerase knock out MEF cells. Cell Signal. 2019, 54, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, D.C.; Bruce, C.R.; Drew, B.G.; Tory, K.; Kolonics, A.; Estevez, E.; Chung, J.; Watson, N.; Gardner, T.; Lee-Young, R.S.; et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 2014, 63, 1881–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, A.; Nishiumi, S.; Yoshida, K.I.; Ashida, H. Rat L6 myotubes as an in vitro model system to study GLUT4-dependent glucose uptake stimulated by inositol derivatives. Cytotechnology 2007, 55, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabo, A.; Sumegi, K.; Fekete, K.; Hocsak, E.; Debreczeni, B.; Setalo, G., Jr.; Kovacs, K.; Deres, L.; Kengyel, A.; Kovacs, D.; et al. Activation of mitochondrial fusion provides a new treatment for mitochondria-related diseases. Biochem. Pharmacol. 2018, 150, 86–96. [Google Scholar] [CrossRef]
- Gutiérrez-Ruiz, M.C.; Bucio, L.; Souza, V.; Gómez, J.J.; Campos, C.; Cárabez, A. Expression of Some Hepatocyte-like Functional Properties of WRL-68 Cells in Culture Society. In vitro Cell Dev. Biol. Anim. 1994, 30, 366–371. [Google Scholar] [CrossRef]
- McMahon, D.K.; Anderson, P.A.; Nassar, R.; Bunting, J.B.; Saba, Z.; Oakeley, A.E.; Malouf, N.N. C2C12 cells: Biophysical, biochemical, and immunocytochemical properties. Am. J. Physiol. 1994, 266, C1795–C1802. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Liu, G.; Jia, Y.; Yang, J.; Shi, J.; Dong, J.; Wei, J.; Liu, X. Characterization of air-liquid interface culture of A549 alveolar epithelial cells. Brazilian J. Med. Biol. Res. 2018, 51, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.H.; Seifert, R. Sf9 cells: A versatile model system to investigate the pharmacological properties of G protein-coupled receptors. Pharmacol. Ther. 2010, 128, 387–418. [Google Scholar] [CrossRef]
- Zordoky, B.N.M.; El-Kadi, A.O.S. H9c2 cell line is a valuable in vitro model to study the drug metabolizing enzymes in the heart. J. Pharmacol. Toxicol. Methods 2007, 56, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kadoi, H.; Yamamuro, A.; Ishimaru, Y.; Maeda, S. Noradrenaline increases intracellular glutathione in human astrocytoma U-251 MG cells by inducing glutamate-cysteine ligase protein via β3-adrenoceptor stimulation. Eur. J. Pharmacol. 2016, 772, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gombos, I.; Crul, T.; Piotto, S.; Güngör, B.; Török, Z.; Balogh, G.; Péter, M.; Slotte, P.J.; Campana, F.; Pilbat, A. Membrane-lipid therapy in operation: The HSP co-inducer BGP-15 activates stress signal transduction pathways by remodeling plasma membrane rafts. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sarszegi, Z.; Bognar, E.; Gaszner, B.; Kónyi, A.; Gallyas, F., Jr.; Sumegi, B.; Berente, Z. BGP-15, a PARP-inhibitor, prevents imatinib-induced cardiotoxicity by activating Akt and suppressing JNK and p38 MAP kinases. Mol. Cell. Biochem. 2012, 365, 129–137. [Google Scholar] [CrossRef]
- Schechter, M.A.; Southerland, K.W.; Feger, B.J.; Linder, D., Jr.; Ali, A.A.; Njoroge, L.; Milano, C.A.; Bowles, D.E. An Isolated Working Heart System for Large Animal Models. J. Vis. Exp. 2014, 88, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, H.; Cacciani, N.; Akkad, H.; Larsson, L. Targeting heat shock proteins mitigates ventilator induced diaphragm muscle dysfunction in an age-dependent manner. Front. Physiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Taconic. Available online: https://www.taconic.com/rat-model/sprague-dawley (accessed on 21 June 2019).
- Oana, F.; Takeda, H.; Hayakawa, K.; Matsuzawa, A.; Akahane, S.; Isaji, M.; Akahane, M. Physiological difference between obese (fa/fa) Zucker rats and lean Zucker rats concerning adiponectin. Metabolism 2005, 54, 995–1001. [Google Scholar] [CrossRef]
- Animal Lab. Available online: http://animalab.eu/products/cd-1r-mouse. (accessed on 5 May 2019).
- Chung, J.; Nguyen, A.; Henstridge, D.C.; Holmes, A.G.; Stanley Chan, M.H.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef] [Green Version]
- Drel, V.R.; Mashtalir, N.; Ilnytska, O.; Shin, J.; Li, F.; Lyzogubov, V.V.; Obrosova, I.G. The leptin-deficient (ob/ob) mouse: A new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes 2006, 55, 3335–3343. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, T.L.; Swiderski, K.; Murphy, K.T.; Gehrig, S.M.; Curl, C.L.; Chandramouli, C.; Febbraio, M.A.; Delbridge, L.M.D.; Koopman, R.; Lynch, G.S. BGP-15 Improves Aspects of the Dystrophic Pathology in mdx and dko Mice with Differing Efficacies in Heart and Skeletal Muscle. Am. J. Pathol. 2016, 186, 3246–3260. [Google Scholar] [CrossRef] [Green Version]
- Isaac, C.; Wright, A.; Usas, A.; Li, H.; Tang, Y.; Mu, X.; Greco, N.; Dong, Q.; Vo, N.; Kang, J.; et al. Dystrophin and utrophin ‘double knockout’ dystrophic mice exhibit a spectrum of degenerative musculoskeletal abnormalities. J. Orthop. Res. 2013, 31, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Harada, M.; Hirota, Y.; Nose, E.; Azhary, J.M.K.; Koike, H.; Kunitomi, C.; Yoshino, O.; Izumi, G.; Hirata, T. Activation of Endoplasmic Reticulum Stress in Granulosa Cells from Patients with Polycystic Ovary Syndrome Contributes to Ovarian Fibrosis. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorensen, J.C.; Petersen, A.C.; Timpani, C.A.; Campelj, D.G.; Cook, J.; Trewin, A.J.; Stojanovska, V.; Stewart, M.; Hayes, A.; Rybalka, E. BGP-15 protects against oxaliplatin-induced skeletal myopathy and mitochondrial reactive oxygen species production in mice. Front. Pharmacol. 2017, 8, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuade, R.M.; Stojanovska, V.; Stavely, R.; Timpani, C.; Petersen, A.C.; Abalo, R.; Bornstein, J.C.; Rybalka, E.; Nurgali, K. Oxaliplatin-induced enteric neuronal loss and intestinal dysfunction is prevented by co-treatment with BGP-15. Br. J. Pharmacol. 2018, 175, 656–677. [Google Scholar] [CrossRef] [Green Version]
- Faanes, R.B.; Merluzzi, V.J.; Williams, N.H.; Tarnowski, G.S. Matching of chemotherapy to mouse strain and lymphoid tumor type to prevent tumor-induced suppression of specific T- and B-cell functions. Cancer Res. 1979, 39, 4564–4574. [Google Scholar]
- Charles River. Available online: https://www.criver.com/products-services/find-model/nmri-mouse?region=3631 (accessed on 14 May 2019).
- Nascimento, T.L.; Silva, M.T.; Miyabara, E.H. BGP-15 improves contractile function of regenerating soleus muscle. J. Muscle Res. Cell Motil. 2018, 39, 25–34. [Google Scholar] [CrossRef]
- The Jackson Laboratory. Available online: https://www.jax.org/strain/100007 (accessed on 14 May 2019).
- Wu, L.L.; Russel, D.L.; Wong, S.L.; Chen, M.; Tsai, T.; St. John, J.C.; Norman, R.J.; Febbraio, M.A.; Carrol, J.; Robker, R.L. Mitochondrial dysfunction in oocytes of obese mothers: Transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Developement 2015, 142, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Xing, B.; Wang, L.; Li, Q.; Cao, Y.; Dong, X.; Liang, Y.; Wu, X. Hsp70 plays an important role in high-fat diet induced gestational hyperglycemia in mice. J. Physiol. Biochem. 2015, 71, 649–658. [Google Scholar] [CrossRef]
- Zhang, D.; Ke, L.; Mackovicova, K.; Johannes, J.L.; Van der Want, J.J.; Sibon, O.C.M.; Tanguay, R.M.; Morrow, G.; Henning, R.H.; Kampinga, H.H.; et al. Effects of different small HSPB members on contractile dysfunction and structural changes in a Drosophila melanogaster model for Atrial Fibrillation. J. Mol. Cell. Cardiol. 2011, 51, 381–389. [Google Scholar] [CrossRef]
- Literáti-Nagy, B.; Péterfai, É.; Kulcsár, E.; Literáti-Nagy, Z.; Buday, B.; Tory, K.; Mandl, J.; Sümegi, B.; Fleming, A.; Roth, J.; et al. Beneficial effect of the insulin sensitizer (HSP inducer) BGP-15 on olanzapine-induced metabolic disorders. Brain Res. Bull. 2010, 83, 340–344. [Google Scholar] [CrossRef]
- Literáti-Nagy, Z.; Tory, K.; Literáti-Nagy, B.; Kolonics, A.; Török, Z.; Gombos, I.; Balogh, G.; Vígh, L., Jr.; Horváth, I.; Mandl, J.; et al. The HSP co-inducer BGP-15 can prevent the metabolic side effects of the atypical antipsychotics. Cell Stress Soc. Int. 2016, 17, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Literati-Nagy, Z.; Tory, K.; Literati-Nagy, B.; Kolonics, A.; Vígh, L., Jr.; Vígh, L.; Mandl, J.; Szilvássy, Z. A novel insulin sensitizer drug candidate-BGP-15-can prevent metabolic side effects of atypical antipsychotics. Pathol. Oncol. Res. 2012, 18, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Cacciani, N.; Salah, H.; Li, M.; Akkad, H.; Backeus, A.; Hedstrom, Y.; Jena, B.P.; Bergquist, J.; Larsson, L. Chaperone co-inducer BGP-15 mitigates early contractile dysfunction of the soleus muscle in a rat ICU model. Acta Physiol. 2019, e13425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappato, R.; Castelvecchio, S.; Ricci, C.; Bianco, E.; Vitali-Serdoz, L.; Gnecchi-Ruscone, T.; Pittalis, M.; De Ambroggi, L.; Baruscotti, M.; Gaeta, M.; et al. Clinical Efficacy of Ivabradine in Patients with Inappropriate Sinus Tachycardia. J. Am. Coll Cardiol. 2012, 60, 1323–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Animal Model | Studied Effect or Disease |
|---|---|
| Wistar rats [11,14,16,19] | insulin sensitizing effect, chemoprotective action, cardioprotective effect |
| Goto-Kakizaki rats [17] | insulin sensitizing effect, diabetes caused CV complications |
| Sprague–Dawley rats [40] | insulin sensitizing effect, the role of BGP-15 in alleviating ventilation-induced diaphragm dysfunction |
| Zucker obese rats [4] | insulin sensitizing effect in combination of rimonabant |
| CD-1 mice [1] | liver injury |
| Leptin deficient (ob/ob) mice [44] | testing the role of Hsp72 effective in the treatment of obesity-induced insulin resistance |
| mdx and dko mice [15,46] | Duchenne muscular dystrophy |
| hairless mice (VAF/plus CRL: hr/hr BR) [20] | photoprotective effect |
| BALB/c mice [48,49,50] | PCOS, oxaliplatin therapy induced skeletal myopathy and intestinal dysfunction |
| NMRI CV1 mice and BD2F1 mice [21] | cisplatin caused nephrotoxicity |
| Hsp110 deficient mice [22] | traumatic brain injury |
| CB6F1 mice [53] | effect of BGP-15 on the contractile function and morphology of regenerating soleus muscles |
| cardiac-specific dnPI3K-Mst1 Tg mice20 and cardiac-specific MURC Tg mice40 [18] | heart failure |
| mouse model of Alstrom syndrome [55] | mitochondrial dysfunction |
| C57BL/6 mice [56] | gestational diabetes mellitus |
| white New Zealand rabbits [14] | insulin sensitizing effect |
| Drosophila melanogaster [57] | tachycardia |
| Main Effect | Dose |
|---|---|
| Reconstruction of diastolic dysfunction [17] | 10 mg/kg BGP-15, per os |
| 100 mg/kg metformin, per os | |
| Amelioration of cardiac function and reduction of arrhythmic episodes [18] | 15 mg/kg BGP-15 |
| 50 µM BGP-15 on the cells | |
| Endogenous HSP overexpression and protection against tachycardia remodeling [57] | 1 mM BGP-15 |
| Reduction of ROS levels and cell injury during ischemia–reperfusion [11] | The medium contained 40 mg/L of BFP-15 |
| Main Effect | Dose |
|---|---|
| Improvement of DMD pathology and extension of lifetime [15] | 15 mg/kg per day, oral gavage |
| Improvement of cardiac pathology in DMD, but skeletal muscle function was not improved in older mdx or dko mice [46] | 15 mg/kg BGP-15 |
| Main Effect | Dose |
|---|---|
| Reduction of the toxic side effects of taxol and cisplatin without compromising their antitumor effect [19] | 50, 100, 200 mg/kg of BGP-15, per os, once daily throughout the experiment |
| 1.5 mg/kg cisplatin, intraperitoneally, once daily, for 5 days | |
| 5 mg/kg taxol, intraperitoneally, every other day for 10 days | |
| Reversal of oxidative damage in the heart, caused by imatinib [38] | 200 µM BGP-15 |
| Prevention of the development of acute renal failure caused by cisplatin treatment [21] | 100, 200 mg/kg BGP-15 shortly before cisplatin treatment |
| Alleviation of oxaliplatin-induced intestinal dysfunction, which eased the gastrointestinal side-effects of chemotherapy [50] | 15 mg/kg BGP-15 |
| 3 mg/kg oxaliplatin |
| Main Effect | Dose |
|---|---|
| Prevention of translocation of AIF (apoptosis inducing factor) and mitochondrial depolarization. [1] | 10, 20, 100, 200 mg/kg BGP-15 |
| 450 mg/kg acetaminophen |
| Main Effect | Dose |
|---|---|
| Reduction of olanzapine-induced insulin resistance [58] | 400 mg of BGP-15 or placebo for 17 days |
| 5 mg of olanzapine for 3 days and 10 mg for 14 days | |
| Increase of insulin sensitivity [3] | 200 mg or 400 mg of BGP-15, or placebo once daily, for 4 weeks |
| Production of better results than metformin and rosiglitazone Increase of insulin sensitivity in combination with a sulfonylurea agent (glibenclamide) Increase of insulin sensitizing effect in cholesterol-fed rabbits, but not in normal rabbits. [14] | 5, 10, 20, 30, or 50 mg/kg of BGP-15 per os 2 mg/kg rosiglitazone per os 100 mg/kg metformin per os 1 mg/kg glibenclamide per os |
| Intensification of insulin sensitizing effect of rimonabant; [4] | 10 mg/kg rimonabant 30 mg/kg rimonabant 3 mg/kg BGP-15 10 mg/kg BGP-15 for 5 days |
| Main Effect | Dose |
|---|---|
| Decrease of the number of sunburn cells in UV radiation exposed skin | 5–20% concentration of BGP-15 in the cream |
| DNA protective effect if applied topically [20] | |
| Beneficial effects in the reduction of the pathological consequences of TBI [22] | 15 mg/kg BGP-15, per os |
| Increasing diaphragm muscle fiber force generation capacity. | 40 mg/kg BGP-15 iv. |
| Decreasing the negative effects of mechanical ventilation on diaphragm muscle function [23] |
| Main Effect | Dose |
|---|---|
| Reduction of interstitial fibrosis and collagen deposition in the ovaries [48] | 3 mg/100g of body weight (BGP-15) |
| Reduction of abnormal weight gain after pregnancy [56] | 100 mg/kg BGP-15 |
| Prevention of ROS-related and inflammatory disease progression [24] | 50 µM BGP-15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pető, Á.; Kósa, D.; Fehér, P.; Ujhelyi, Z.; Sinka, D.; Vecsernyés, M.; Szilvássy, Z.; Juhász, B.; Csanádi, Z.; Vígh, L.; et al. Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome. Molecules 2020, 25, 429. https://doi.org/10.3390/molecules25020429
Pető Á, Kósa D, Fehér P, Ujhelyi Z, Sinka D, Vecsernyés M, Szilvássy Z, Juhász B, Csanádi Z, Vígh L, et al. Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome. Molecules. 2020; 25(2):429. https://doi.org/10.3390/molecules25020429
Chicago/Turabian StylePető, Ágota, Dóra Kósa, Pálma Fehér, Zoltán Ujhelyi, Dávid Sinka, Miklós Vecsernyés, Zoltán Szilvássy, Béla Juhász, Zoltán Csanádi, László Vígh, and et al. 2020. "Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome" Molecules 25, no. 2: 429. https://doi.org/10.3390/molecules25020429
APA StylePető, Á., Kósa, D., Fehér, P., Ujhelyi, Z., Sinka, D., Vecsernyés, M., Szilvássy, Z., Juhász, B., Csanádi, Z., Vígh, L., & Bácskay, I. (2020). Pharmacological Overview of the BGP-15 Chemical Agent as a New Drug Candidate for the Treatment of Symptoms of Metabolic Syndrome. Molecules, 25(2), 429. https://doi.org/10.3390/molecules25020429