Advances in Azorella glabra Wedd. Extract Research: In Vitro Antioxidant Activity, Antiproliferative Effects on Acute Myeloid Leukemia Cells and Bioactive Compound Characterization
Abstract
:1. Introduction
2. Results
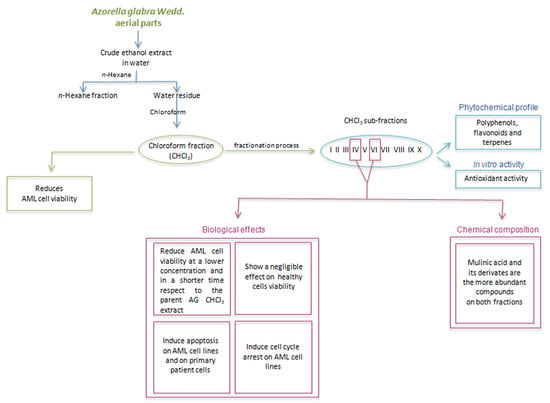
2.1. Viability Analysis of AML Cells Treated with AG CHCl3 Fraction
2.2. Fractionation, Phytochemical Profiles, and In Vitro Antioxidant Activity of AG CHCl3 Sub-Fractions
2.3. Viability Analysis of AML and Healthy Cells Treated with AG CHCl3 Sub-Fractions
2.4. Evaluation of Apoptosis and Cell Cycle in KG1 and MV4-11 Cells Treated with AG IV and VI Sub-Fractions
2.5. Evaluation of Apoptosis and Cell Cycle in Primary AML Cells Treated with AG IV and VI Sub-Fractions
2.6. Phytochemical Identification of AG CHCl3 IV and VI Sub-Fractions
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and Instruments
4.2. Chromatographic Separation of AG CHCl3 Fraction
4.3. Determination of Total Polyphenol Content (TPC), Total Flavonoid Content (TFC), and Total Terpenoid Content (TTeC)
4.4. Determination of Antioxidant Activities
4.5. AG samples Preparation
4.6. Cell Lines, Healthy Donors and AML Patients
4.7. Cell Viability Assay
4.8. Apoptosis Assay
4.9. Cell Cycle Analysis
4.10. LC-MS/MS Characterization of Samples
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural products and acute myeloid leukemia: A review highlighting mechanisms of action. Nutrients 2019, 11, 1010. [Google Scholar] [CrossRef] [Green Version]
- Lamorte, D.; Faraone, I.; Laurenzana, I.; Milella, L.; Trino, S.; De Luca, L.; Del Vecchio, L.; Armentano, M.F.; Sinisgalli, C.; Chiummiento, L.; et al. Future in the past: Azorella glabra Wedd. as a source of new natural compounds with antiproliferative and cytotoxic activity on multiple myeloma cells. Int. J. Mol. Sci. 2018, 19, 3348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Ra, J.-H.; Jee, Y.; Kim, J.-S. Impact of different partitioned solvents on chemical composition and bioavailability of Sasa quelpaertensis Nakai leaf extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahmani, F.; Esmaeili, S.; Bashash, D.; Dehghan-Nayeri, N.; Mashati, P.; Gharehbaghian, A. Centaurea albonitens extract enhances the therapeutic effects of Vincristine in leukemic cells by inducing apoptosis. Biomed. Pharmacother. 2018, 99, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Marzouni, H.Z. Effective medicinal plant in cancer treatment, part 2: Review study. J. Evid. Based Integr. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Jain, S.; Dwivedi, J.; Jain, P.K.; Satpathy, S.; Patra, A. Medicinal plants for treatment of cancer: A brief review. Pharmacogn. J. 2016, 8, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Faraone, I.; Rai, D.K.; Russo, D.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Milella, L. Antioxidant, antidiabetic, and anticholinesterase activities and phytochemical profile of Azorella glabra Wedd. Plants 2019, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Altemimi, A.B.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Ansari, I.A.; Akhtar, M.S. Current insights on the role of terpenoids as anticancer agents: A perspective on cancer prevention and treatment. In Natural Bio-Active Compounds; Springer Science and Business Media LLC: Singapore, 2019; pp. 53–80. [Google Scholar]
- Sung, M.H.; Kwon, O.-K.; Oh, S.-R.; Lee, D.K.; Park, S.-H.; Han, S.-B.; Ahn, K.-S. Azorella compacta methanolic extract induces apoptosis via activation of mitogen-activated protein kinase. Mol. Med. Rep. 2015, 12, 6821–6828. [Google Scholar] [CrossRef]
- Cheel, J.; Tůmová, L.; Dučaiová, Z.; Vokřál, I.; Sepúlveda, B.; Vokurková, D. Azorella compacta infusion activates human immune cells and scavenges free radicals in vitro. Pharmacogn. Mag. 2017, 13, 260–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, A.N.; Plunkett, G.M. Untangling generic limits in Azorella, Laretia, and Mulinum (Apiaceae: Azorelloideae): Insights from phylogenetics and biogeography. Taxon 2012, 61, 826–840. [Google Scholar] [CrossRef]
- Plunkett, G.M.; Nicolas, A.N. Assessing Azorella (Apiaceae) and its allies: Phylogenetics and a new classification. Brittonia 2016, 69, 31–61. [Google Scholar] [CrossRef]
- Fernandez, E.; Sandi, Y.; Kokoska, L. Ethnobotanical inventory of medicinal plants used in the Bustillo Province of the Potosi Department, Bolivia. Fitoterapia 2003, 74, 407–416. [Google Scholar] [CrossRef]
- Cussy-Poma, V.; Fernández, E.; Rondevaldova, J.; Foffová, H.; Russo, D. Ethnobotanical inventory of medicinal plants used in the Qampaya District, Bolivia. Bol. Latinoam. Caribe Plantas Med. Aromat. 2017, 16, 68–77. [Google Scholar]
- Faraone, I.; Rai, D.K.; Chiummiento, L.; Cusimamani, E.F.; Choudhary, A.; Prinzo, F.; Milella, L. Antioxidant activity and phytochemical characterization of Senecio clivicolus Wedd. Molecules 2018, 23, 2497. [Google Scholar] [CrossRef] [Green Version]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Salvia, A.M.; Cuviello, F.; Coluzzi, S.; Nuccorini, R.; Attolico, I.; Pascale, S.P.; Bisaccia, F.; Pizzuti, M.; Ostuni, A. Expression of some ATP-binding cassette transporters in acute myeloid leukemia. Hematol. Rep. 2017, 9, 7406. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, R.; Albanesi, J.; Ascenzi, P.; Nervi, C.; Di Masi, A. Are DNA damage response kinases a target for the differentiation treatment of acute myeloid leukemia? IUBMB Life 2018, 70, 1057–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiggers, C.R.; Baak, M.L.; Sonneveld, E.; Nieuwenhuis, E.E.; Bartels, M.; Creyghton, M.P. AML Subtype is a major determinant of the association between prognostic gene expression signatures and their clinical significance. Cell Rep. 2019, 28, 2866–2877.e5. [Google Scholar] [CrossRef] [PubMed]
- Juliusson, G.; Antunovic, P.; Derolf, Å.; Lehmann, S.; Möllgård, L.; Stockelberg, D.; Tidefelt, U.; Wahlin, A.; Höglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunanithi, S.; Liu, R.; Roe, A.; Moreton, S.; Hou, Y.; Oldford, N.; Goyco, J.V.; Wald, D. Abstract 382: Inhibition of mitochondrial spare respiratory capacity by new securinine derivatives as a novel differentiation therapy for acute myeloid leukemia. Mol. Cell. Biol. 2018, 78, 382. [Google Scholar] [CrossRef]
- Kabeel, M.; Ghoneim, A.M.; Mansy, S.E. Anti-leukemic activity of a four-plant mixture in a leukemic rat model. J. Basic Appl. Zoöl. 2018, 79, 7. [Google Scholar] [CrossRef] [Green Version]
- Pająk, B.; Gajkowska, B.; Orzechowski, A. Molecular basis of parthenolide-dependent proapoptotic activity in cancer cells. Folia Histochem. Cytobiol. 2008, 46, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Areche, C.; Fernandez-Burgos, R.; De Terrones, T.C.; Simirgiotis, M.; García-Beltrán, O.; Borquez, J.; Sepulveda, B. Mulinum crassifolium Phil; two new mulinanes, gastroprotective activity and metabolomic analysis by UHPLC-orbitrap mass spectrometry. Molecules 2019, 24, 1673. [Google Scholar] [CrossRef] [Green Version]
- Wächter, G.A.; Matooq, G.; Hoffmann, J.J.; Maiese, W.M.; Singh, M.P.; Montenegro, G.; Timmermann, B.N. Antibacterial diterpenoid acids from Azorella compacta. J. Nat. Prod. 1999, 62, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Kong, M.; Pizarro, E.; Pasten, C.; Morales, G.; Borquez, J.; Loyola, L.A. Effect of Azorellanone, a Diterpene from Azorella yareta Hauman, on human sperm physiology. J. Androl. 2003, 24, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.B.; Pappano, D.B.; Bustos, D.A.; Sosa, V.E.; Baggio, R.F.; Garland, M.T.; Gil, R.R. Azorellane diterpenes from Azorella cryptantha. Phytochemistry 2004, 65, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Bórquez, J.; Bartolucci, N.L.; Echiburú-Chau, C.; Winterhalter, P.; Vallejos, J.; Jerz, G.; Simirgiotis, M.J. Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama desert by high-speed counter-current chromatography. J. Sci. Food Agric. 2015, 96, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-R.; Chang, C.; Hsu, C.; Tsai, M.; Cheng, H.; Leong, M.K.; Sung, P.; Chen, J.; Weng, C.-F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2019, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- San-Martin, A.; Donoso, V.; Leiva, S.; Bacho, M.; Nunez, S.; Gutierrez, M.; Rovirosa, J.; Bailon-Moscoso, N.; Camacho, S.C.; Aviles, O.M.; et al. Molecular docking studies of the antitumoral activity and characterization of new chalcone. Curr. Top. Med. Chem. 2015, 15, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Patel, M.; Reddy, M.N.; Alshammari, E. Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dutt, R.; Garg, V.; Khatri, N.; Madan, A.K. Phytochemicals in anticancer drug development. Anti-Cancer Agents Med. Chem. 2019, 19, 172–183. [Google Scholar] [CrossRef]
- Ervina, M.; Sukardiman. A review: Melia azedarach L. as a potent anticancer drug. Pharmacogn. Rev. 2018, 12, 94–102. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Ehussain, G.; Huang, J.; Rasul, A.; Anwar, H.; Imran, A.; Maqbool, J.; Razzaq, A.; Aziz, N.; Makhdoom, E.U.H.; Konuk, M.; et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: An updated review. Molecules 2019, 24, 2213. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.-C.; Tsai, C.-M.; Lu, C.-C.; Weng, C.-J. Recent progress in natural dietary non-phenolic bioactives on cancers metastasis. J. Food Drug Anal. 2018, 26, 940–964. [Google Scholar] [CrossRef] [PubMed]
- Grigalius, I.; Petrikaitė, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonam, K.S.; Guleria, S. Synergistic antioxidant activity of natural products. Ann. Pharmacol. Pharm. 2017, 2, 1–6. [Google Scholar]
- Wang, T.Y.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Sohail, A.; Farhan, M.; Rehman, A.A.; Ahmad, A.; Hadi, S. Flavonoids-induced redox cycling of copper ions leads to generation of reactive oxygen species: A potential role in cancer chemoprevention. Int. J. Biol. Macromol. 2018, 106, 569–578. [Google Scholar] [CrossRef]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.D.M.; Cadena-Zamudio, J.D.; González-Ugarte, A.K.; Weiss Steider, B.; Santiago-Osorio, E. Fruit extract from a Sechium edule hybrid induce apoptosis in leukaemic cell lines but not in normal cells. Nutr. Cancer 2015. [Google Scholar] [CrossRef]
- Dahham, S.S.; Hassan, L.E.A.; Ahamed, M.B.K.; Majid, A.S.A.; Majid, A.M.S.A.; Noriman, N.Z. In vivo toxicity and antitumor activity of essential oils extract from agarwood (Aquilaria crassna). BMC Complement. Altern. Med. 2016, 16, 236. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wang, X.; Hu, D. Furanodienone induces G0/G1 arrest and causes apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway in human colorectal cancer cells: A study in vitro and in vivo. Cell Death Dis. 2017, 8, e2815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Kumar, Y. Cancer immunoediting: Immunosurveillance, immune equilibrium, and immune escape. In Cancer Immunology: A Translational Medicine Context; Springer: Berlin, Heidelberg, 2015; pp. 195–208. ISBN 9783662440063. [Google Scholar]
- Chu, H.; Li, M.; Wang, X. Capsaicin induces apoptosis and autophagy in human melanoma cells. Oncol. Lett. 2019, 17, 4827–4834. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2017, 25, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Cui, C.; Chen, C.-Q.; Hu, X.-L.; Liu, Y.-H.; Fan, Y.-H.; Meng, W.-H.; Zhao, Q.-C. Anti-proliferative and pro-apoptotic activities of Alpinia oxyphylla on HepG2 cells through ROS-mediated signaling pathway. J. Ethnopharmacol. 2015, 169, 99–108. [Google Scholar] [CrossRef]
- Hao, G.P.; Zhao, F.X.; Zhang, Y.Y.; Yang, Z.F. Acetoacetate extract of Pleione bulbocodioides (Franch.) rolfe induces apoptosis of human leukemia thp-1cells through a mitochondria-regulated intrinsic apoptotic pathway. Biosci. J. 2019, 609–619. [Google Scholar] [CrossRef] [Green Version]
- El Khoury, M.; Haykal, T.; Hodroj, M.H.; Najem, S.A.; Sarkis, R.; Taleb, R.I.; Rizk, S. Malva Pseudolavatera leaf extract promotes ROS induction leading to apoptosis in acute myeloid leukemia cells in vitro. Cancers 2020, 12, 435. [Google Scholar] [CrossRef] [Green Version]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- McGill, C.M.; Tomco, P.L.; Ondrasik, R.M.; Belknap, K.C.; Dwyer, G.K.; Quinlan, D.J.; Kircher, T.A.; Andam, C.P.; Brown, T.J.; Claxton, D.F.; et al. Therapeutic effect of northern labrador tea extracts for acute myeloid leukemia. Phytother. Res. 2018, 32, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Simoneit, B.R.; Oros, D.R.; Jaffé, R.; Didyk-Peña, A.; Areche, C.; Sepúlveda, B.; Didyk, B.M. Mulinane and Azorellane diterpenoid biomarkers by GC-MS from a representative Apiaceae (Umbelliferae) species of the Andes. Molecules 2019, 24, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Salinas, G.M.; Borquez, J.; Ardiles, A.; Said-Fernández, S.; Loyola, L.A.; Yam-Puc, A.; Becerril-Montes, P.; Escalante-Erosa, F.; San-Martin, A.; González-Collado, I.; et al. Bioactive metabolites from the Andean flora. Antituberculosis activity of natural and semisynthetic azorellane and mulinane diterpenoids. Phytochem. Rev. 2010, 9, 271–278. [Google Scholar] [CrossRef]
- Laurenzana, I.; Caivano, A.; Trino, S.; De Luca, L.; La Rocca, F.; Simeon, V.; Tintori, C.; D’Alessio, F.; Teramo, A.; Zambello, R.; et al. A pyrazolo [3,4-d] pyrimidine compound inhibits Fyn phosphorylation and induces apoptosis in natural killer cell leukemia. Oncotarget 2016, 7, 65171–65184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Y.; Li, C. Simultaneous determination of ginkgo flavonoids and terpenoids in plasma: Ammonium formate in LC mobile phase enhancing electrospray ionization efficiency and capacity. J. Am. Soc. Mass Spectrom. 2008, 19, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sora, D.I.; Stefanescu, V.; David, V.; Medvedovici, A. Validation of an LC–MS/MS assay of terpene trilactones in Ginkgo biloba extracts and pharmaceutical formulations through standard addition method. J. Pharm. Biomed. Anal. 2009, 50, 459–468. [Google Scholar] [CrossRef] [PubMed]








| Cell Lines | EC50 (μg/mL) | |
|---|---|---|
| 24 h | 48 h | |
| KG1 | 45.42 | 39.90 |
| MV4-11 | 49.00 | 46.53 |
| Samples | ABTS (mgTE/g) | SO (IC25 μg/mL) |
|---|---|---|
| AG CHCl3 | 32.08 ± 0.02 a | 470.00 ± 20.00 c |
| I | nd | 188.72 ± 16.68 b |
| II | 22.85 ± 0.57 b | 46.31 ± 2.05 a,b |
| III | 15.14 ± 0.64 c | 1996.03 ± 97.23 e |
| IV | 13.08 ± 0.22 d | 2171.71 ± 125.59 f |
| V | 17.86 ± 1.05 e | 67.78 ± 1.80 a,b |
| VI | 12.48 ± 0.43 d | 115.41 ± 0.85 a,b |
| VII | 23.25 ± 0.72 b | 1174.48 ± 43.23 d |
| VIII | 11.65 ± 0.55 d | 52.08 ± 2.38 a,b |
| IX | 16.40 ± 0.50 c,e | 35.96 ± 3.04 a |
| X | 17.70 ± 0.64 e | 54.80 ± 4.47 a,b |
| Fraction | Cell Lines | EC50 (μg/mL) | |
|---|---|---|---|
| 24 h | 48 h | ||
| IV | KG1 | 35.40 | 27.60 |
| MV4-11 | 28.50 | 8.99 | |
| VI | KG1 | 33.72 | 30.40 |
| MV4-11 | 32.91 | 9.87 | |
| Peak No. | Retention Time (min) | ESI (-) MS Observed | ESI (-) MS Calc. | Molecular Formula | Δppm | MS/MS | Tentative Identity |
|---|---|---|---|---|---|---|---|
| 1 | 12.36 | 333.2072 | 333.2071 | C20H30O4 | 0.3 | 289, 287, 271, 253, 229, 135, 113, 95, 87, 83, 69, 57 | Mulinic acid or 14α-hydroxymulin-12-en-11-one-20-oic acid [25] |
| 2 | 13.40 | 333.2072 | 333.2071 | C20H30O4 | 0.3 | 289, 287, 271, 253, 229, 135, 113, 95, 87, 83, 69, 57 | Mulinic acid or 14α-hydroxymulin-12-en-11-one-20-oic acid [25] |
| 3 | 13.94 | 331.1917 | 331.1915 | C20H28O4 | 0.6 | 287, 269, 259, 243, 229, 215, 121, 111, 109, 96, 83 | Mulin-12-en-11, 14-dion-20-oic acid [26] |
| 4 | 14.43 | 303.1945 | 303.2345 | C20H32O2 | 4.9 | 259, 249, 243, 217, 189, 135, 123, 95, 81, 57 | Azorellanone 33 or Dihydroazorellolide [28] |
| 5 | 16.61 | 497.2734 | 497.2756 | C26H42O9 | −4.4 | 333, 271, 229, 135, 113, 95, 83, 69, 57 | Mulinic acid hexoside derivative |
| Peak No. | Retention Time (min) | ESI (-) MS Observed | ESI (-) MS Calc. | Molecular Formula | Δppm | MS/MS | Tentative Identity |
|---|---|---|---|---|---|---|---|
| 1 | 10.76 | 335.2238 | 335.2230 | C20H32O4 | 2.4 | 289, 273, 245, 235, 59 | 13,14-dihydroxymulin-11-en-20-oic acid [29] |
| 2 | 10.97 | 335.2238 | 335.2230 | C20H32O4 | 2.4 | 289, 273, 245, 235, 59 | 13,14-dihydroxymulin-11-en-20-oic acid [29] |
| 3 | 11.26 | 349.2368 | 349.2384 | C21H34O4 | −4.6 | 331, 305, 287, 269, 259, 245, 235, 219, 205, 193, 177, 151, 123, 111, 99, 83, 69, 59, 57 | Mulin-en-dion-oicacid derivative |
| 4 | 11.53 | 335.2238 | 335.2230 | C20H32O4 | 2.4 | 289, 273, 245, 235, 59 | 13,14-dihydroxymulin-11-en-20-oic acid [29] |
| 5 | 12.19 | 393.2427 | 393.2430 | C26H34O3 | −0.8 | 353, 335, 289, 273, 245, 235, 59 | Dihydroxymulinic acid derivative |
| 6 | 12.74 | 333.2072 | 333.2071 | C20H30O4 | 0.3 | 289, 287, 271, 253, 229, 135, 113, 95, 87, 83, 69, 57 | Mulinic acid or 14α-hydroxymulin-12-en-11-one-20-oic acid [25] |
| 7 | 13.90 | 331.1917 | 331.1915 | C20H28O4 | 0.6 | 287, 269, 259, 243, 229, 215, 121, 111, 109, 96, 83 | Mulin-12-en-11, 14-dion-20-oic acid [26] |
| AML Patients | Age | Sex | Mutation | Blast (%) |
|---|---|---|---|---|
| 1 | 68 | Female | FLT3-ITD NPM1 | >90 |
| 2 | 78 | Female | NPM1 | 60–70 |
| 3 | 58 | Female | FLT3-ITD NPM1 | 100 |
Sample Availability: Samples of the compounds used for this study are available from the authors on request. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamorte, D.; Faraone, I.; Laurenzana, I.; Trino, S.; Russo, D.; Rai, D.K.; Armentano, M.F.; Musto, P.; Sgambato, A.; De Luca, L.; et al. Advances in Azorella glabra Wedd. Extract Research: In Vitro Antioxidant Activity, Antiproliferative Effects on Acute Myeloid Leukemia Cells and Bioactive Compound Characterization. Molecules 2020, 25, 4890. https://doi.org/10.3390/molecules25214890
Lamorte D, Faraone I, Laurenzana I, Trino S, Russo D, Rai DK, Armentano MF, Musto P, Sgambato A, De Luca L, et al. Advances in Azorella glabra Wedd. Extract Research: In Vitro Antioxidant Activity, Antiproliferative Effects on Acute Myeloid Leukemia Cells and Bioactive Compound Characterization. Molecules. 2020; 25(21):4890. https://doi.org/10.3390/molecules25214890
Chicago/Turabian StyleLamorte, Daniela, Immacolata Faraone, Ilaria Laurenzana, Stefania Trino, Daniela Russo, Dilip K. Rai, Maria Francesca Armentano, Pellegrino Musto, Alessandro Sgambato, Luciana De Luca, and et al. 2020. "Advances in Azorella glabra Wedd. Extract Research: In Vitro Antioxidant Activity, Antiproliferative Effects on Acute Myeloid Leukemia Cells and Bioactive Compound Characterization" Molecules 25, no. 21: 4890. https://doi.org/10.3390/molecules25214890
APA StyleLamorte, D., Faraone, I., Laurenzana, I., Trino, S., Russo, D., Rai, D. K., Armentano, M. F., Musto, P., Sgambato, A., De Luca, L., Milella, L., & Caivano, A. (2020). Advances in Azorella glabra Wedd. Extract Research: In Vitro Antioxidant Activity, Antiproliferative Effects on Acute Myeloid Leukemia Cells and Bioactive Compound Characterization. Molecules, 25(21), 4890. https://doi.org/10.3390/molecules25214890