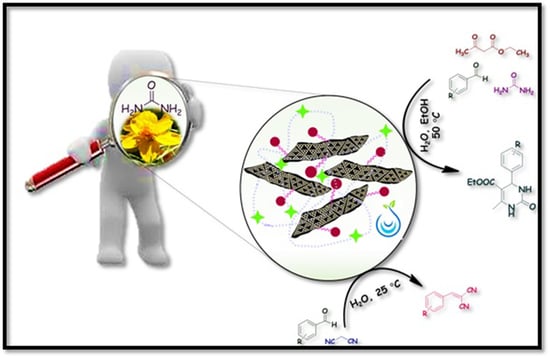
Biochar-Based Graphitic Carbon Nitride Adorned with Ionic Liquid Containing Acidic Polymer: A Versatile, Non-Metallic Catalyst for Acid Catalyzed Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation of Formation of BC@GCN-P-IL
2.2. Catalyst Activity
2.3. Appraising the Contribution of Catalyst Moieties to the Catalysis
2.4. Recyclability of BC@GCN-P-IL
3. Materials and Methods
3.1. Apparatus
3.2. Synthesis of the Catalyst
3.2.1. Synthesis of BC@GCN
3.2.2. Synthesis of BC@GCN-V
3.2.3. Synthesis of BC@GCN-P-IL
3.3. Evaluation of the Catalytic Activity
3.3.1. Knoevenagel Condensation Reaction
3.3.2. Biginelli Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karimi, F.; Zolfigol, M.A.; Yarie, M. A novel and reusable ionically tagged nanomagnetic catalyst: Application for the preparation of 2-amino-6-(2-oxo-2H-chromen-3-yl)-4-arylnicotinonitriles via vinylogous anomeric based oxidation. Mol. Catal. 2019, 463, 20–29. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Gholamhosseini-Nazari, M.; Payami, E.; Esmati, S. Ferrocene-tagged ionic liquid stabilized on silica-coated magnetic nanoparticles: Efficient catalyst for the synthesis of 2-amino-3-cyano-4H-pyran derivatives under solvent-free conditions. Appl. Organomet. Chem. 2018, 32, e3955. [Google Scholar] [CrossRef]
- Rafiee, E.; Kahrizi, M. Mechanistic investigation of Heck reaction catalyzed by new catalytic system composed of Fe3O4@OA–Pd and ionic liquids as co-catalyst. J. Mol. Liq. 2016, 218, 625–631. [Google Scholar] [CrossRef]
- Sadjadi, S.; Akbari, M.; Heravi, M.M. Palladated Nanocomposite of Halloysite–Nitrogen-Doped Porous Carbon Prepared from a Novel Cyano-/Nitrile-Free Task Specific Ionic Liquid: An Efficient Catalyst for Hydrogenation. ACS Omega 2019, 4, 19442–19451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öztürk, B.Ö. Ammonium tagged Hoveyda-Grubbs catalysts immobilized on magnetically separable core-shell silica supports for ring-closing metathesis reactions. Microporous Mesoporous Mater. 2018, 267, 249–256. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Malmir, M.; Kahangi, F.G. Ionic-liquid and cuprous sulfite containing halloysite nanoclay: An efficient catalyst for Click reaction as well as N- and O-arylations. Appl. Clay Sci. 2018, 162, 192–203. [Google Scholar] [CrossRef]
- Sadjadi, S.; Akbari, M.; Monflier, E.; Heravi, M.M.; Leger, B. Pd nanoparticles immobilized on halloysite decorated with a cyclodextrin modified melamine-based polymer: A promising heterogeneous catalyst for hydrogenation of nitroarenes. New J. Chem. 2018, 42, 15733–15742. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F. Functionalized chitosan polymerized with cyclodextrin decorated ionic liquid: Metal free and biocompatible catalyst for chemical transformations. Int. J. Biol. Macromol. 2020, 147, 399–407. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef]
- Sun, S.; Gou, X.; Tao, S.; Cui, J.; Li, J.; Yang, Q.; Liang, S.; Yang, Z. Mesoporous graphitic carbon nitride (gC3N4) nanosheets synthesized from carbonated beverage-reformed commercial melamine for enhanced photocatalytic hydrogen evolution. Mater. Chem. Front. 2019, 3, 597–605. [Google Scholar] [CrossRef]
- Lau, V.W.-h.; Moudrakovski, I.; Botari, T.; Weinberger, S.; Mesch, M.B.; Duppel, V.; Senker, J.; Blum, V.; Lotsch, B.V. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat. Commun. 2016, 7, 12165. [Google Scholar] [CrossRef] [PubMed]
- Denisov, N.; Chubenko, E.; Bondarenko, V.; Borisenko, V. Synthesis of Oxygen-Doped Graphitic Carbon Nitride from Thiourea. Tech. Phys. Lett. 2019, 45, 108–110. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Nehra, S.; Sharma, A. Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 2019, 9, 15381–15391. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Reddy, C.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications. J. Environ. Manag. 2019, 238, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, S.-X.; Yin, W.-J.; Sheng, W.; Wu, L.-X.; Nie, G.-Z.; Ao, Z. Adsorption behaviors of HCN, SO2, H2S and NO molecules on graphitic carbon nitride with Mo atom decoration. Appl. Surf. Sci. 2020, 501, 144199. [Google Scholar] [CrossRef]
- Subhan, F.; Khan, I.; Hong, J. Two-dimensional graphitic carbon nitride (g-C4N3) for superior selectivity of multiple toxic gases (CO, NO2, and NH3). Nanotechnology 2020, 31, 145501. [Google Scholar] [CrossRef] [PubMed]
- Ingabire, P.B.; Pan, X.; Haragirimana, A.; Li, N.; Hu, Z.; Chen, S. Enhanced conduction capability of nanocomposite membrane of quaternized poly (arylene ether sulfone) s covalently bonded with graphitic carbon nitride nanosheets for fuel cells. React. Funct. Polym. 2019, 144, 104260. [Google Scholar] [CrossRef]
- Dorraj, M.; Sadjadi, S.; Heravi, M.M. Pd on poly(1-vinylimidazole) decorated magnetic S-doped grafitic carbon nitride: An efficient catalyst for catalytic reduction of organic dyes. Sci. Rep. 2020, 10, 13440. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Xie, Z.-L.; Su, D.S. Ionic Liquid Based Approaches to Carbon Materials Synthesis. Eur. J. Inorg. Chem. 2015, 2015, 1137–1147. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Y.; Li, Y. Utilization of corn cob biochar in a direct carbon fuel cell. J. Power Sources 2014, 270, 312–317. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Z.; Liu, Y.C.; Dai, B.; Zou, Y.; Gong, X.; Wang, Y.; Deng, X.; Wu, H.; Xu, Q.; et al. Ionic liquid-functionalized biochar sulfonic acid as a biomimetic catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Green Chem. 2012, 14, 1928–1934. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Yan, Z.; Tan, X.; Liu, S.; Zeng, G.; Gu, Y.; Hu, X.; Jiang, L. Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J. Chem. Technol. Biotechnol. 2018, 93, 783–791. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Liu, S.; Tan, X.; Ding, Y.; Zeng, G.; Zhou, Y.; Zhang, M.; Wang, S.; Zheng, B.; et al. Effective removal of Cr(VI) using b-cyclodextrin–chitosan modified biochars with adsorption/reduction bifuctional roles. RSC Adv. 2016, 6, 94–104. [Google Scholar] [CrossRef]
- Liu, S.; Huang, B.; Chai, L.; Liu, Y.; Zeng, G.; Wang, X.; Zeng, W.; Shang, M.; Deng, J.; Zhou, Z. Enhancement of As(V) adsorption from aqueous solution by a magnetic chitosan/biochar composite. RSC Adv. 2017, 7, 10891–10900. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, X.; Zhu, Y.; Wang, L.; Wang, Z. In situ preparation of biochar coated silica material from rice husk. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 157–160. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, E.S.M.; Martin-Aranda, R.; Yeung, K.L. An investigation of Knoevenagel condensation reaction in microreactors using a new zeolite catalyst. Appl. Catal. A 2004, 261, 109–118. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Niu, Y.; Wang, G.; Li, Y.; Zhao, Y.; Singh, V.; Niu, J.; Wang, J. Polyoxoniobates as a superior Lewis base efficiently catalyzed Knoevenagel condensation. Mol. Catal. 2018, 453, 93–99. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.; Jiang, P.; Gao, W.; Cong, R.; Yang, T. Structure-induced Lewis-base Ga4B2O9 and its superior performance in Knoevenagel condensation reaction. Mol. Catal. 2020, 490, 110914. [Google Scholar] [CrossRef]
- Al-Shehri, B.M.; Shabaan, M.R.; Shkir, M.; Kaushik, A.; Hamdy, M.S. Single-step fabrication of Na-TUD-1 novel heterogeneous base nano-catalyst for Knoevenagel condensation reaction. J. Nanostrucure Chem. 2020, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Jin, Y.; Li, T. Two PbII-based coordination polymers based on 5-aminonicotinic acid and 5-hydroxynicotinic acid for Knoevenagel condensation reaction and luminescent sensor. J. Solid State Chem. 2019, 278, 120927. [Google Scholar] [CrossRef]
- Burate, P.A.; Javle, B.R.; Desale, P.H.; Kinage, A.K. Amino acid amide based ionic liquid as an efficient organo-catalyst for solvent-free Knoevenagel condensation at room temperature. Catal. Lett. 2019, 149, 2368–2375. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Huang, X.; Zhang, M.; Bian, G.; Qi, Y.; Yang, X.; Zhang, W. Mesoporous polymeric catalysts with both sulfonic acid and basic amine groups for the one-pot deacetalization–Knoevenagel reaction. New J. Chem. 2019, 43, 16676–16684. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Z.; Xu, Q.; Han, X.; Ma, X.; Wang, J.; Kannan, T.; Ma, P.; Wang, J.; Niu, J. Polyoxomolybdates as efficient catalysts for Knoevenagel condensation reaction of benzaldehyde and ethyl cyanoacetate under mild condition. J. Mater. Sci. 2021, 56, 4654–4665. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Halloysite Nanotubes Loaded with Calcium Hydroxide: Alkaline Fillers for the Deacidification of Waterlogged Archeological Woods. ACS Appl. Mater. Interfaces 2018, 10, 27355–27364. [Google Scholar] [CrossRef]
- Luan, Y.; Qi, Y.; Gao, H.; Andriamitantsoa, R.S.; Zheng, N.; Wang, G. A general post-synthetic modification approach of amino-tagged metal–organic frameworks to access efficient catalysts for the Knoevenagel condensation reaction. J. Mater. Chem. A 2015, 3, 17320. [Google Scholar] [CrossRef]
- Zaheri, H.M.; Javanshir, S.; Hemmati, B.; Dolatkhah, Z.; Fardpour, M. Magnetic core–shell Carrageenan moss/Fe3O4: A polysaccharide-based metallic nanoparticles for synthesis of pyrimidinone derivatives via Biginelli reaction. Chem. Cent. J. 2018, 12, 108. [Google Scholar] [CrossRef]
- Kargar, S.; Elhamifar, D.; Zarnegaryan, A. Core–shell structured Fe3O4@ SiO2-supported IL/[Mo6O19]: A novel and magnetically recoverable nanocatalyst for the preparation of biologically active dihydropyrimidinones. J. Phys. Chem. Solids 2020, 146, 109601. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Biginelli reaction on Fe3O4–MWCNT nanocomposite: Excellent reactivity and facile recyclability of the catalyst combined with ultrasound irradiation. RSC Adv. 2013, 3, 17962–17967. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F. Bentonite with high loading of ionic liquid: A potent non-metallic catalyst for the synthesis of dihydropyrimidinones. J. Mol. Liq. 2020, 319, 114393. [Google Scholar] [CrossRef]
- Mashhoori, M.-S.; Sandaroos, R.; Moghaddam, A.Z. Polymeric imidazolium ionic liquid-tagged manganese Schiff base complex: An efficient catalyst for the Biginelli reaction. Res. Chem. Intermed. 2020, 46, 4939–4954. [Google Scholar] [CrossRef]
- Azizi, N.; Edrisi, M. Preparation of choline sulfate ionic liquid supported on porous graphitic carbon nitride nanosheets by simple surface modification for enhanced catalytic properties. J. Mol. Liq. 2020, 300, 112263. [Google Scholar] [CrossRef]
- Liberto, N.A.; de Paiva Silva, S.; de Fátima, Â.; Fernandes, S.A. β-Cyclodextrin-assisted synthesis of Biginelli adducts under solvent-free conditions. Tetrahedron 2013, 69, 8245–8249. [Google Scholar] [CrossRef]
- Kamalzare, P.; Mirza, B.; Soleimani-Amiri, S. Chitosan magnetic nanocomposite: A magnetically reusable nanocatalyst for green synthesis of Hantzsch 1, 4-dihydropyridines under solvent-free conditions. J. Nanostructure Chem. 2020, 1–15. [Google Scholar] [CrossRef]
- Doustkhah, E.; Rostamnia, S.; Hassankhani, A. The raise of SBA-SO 3 H catalytic activity by inducing ultrasound irradiation in the multicomponent syntheses. J. Porous Mater. 2016, 23, 549–556. [Google Scholar] [CrossRef]
- Xin, J.; Chang, L.; Hou, Z.; Shang, D.; Liu, X.; Feng, X. An enantioselective Biginelli reaction catalyzed by a simple chiral secondary amine and achiral Brønsted acid by a dual-activation route. Chem. Eur. J. 2008, 14, 3177–3181. [Google Scholar] [CrossRef]
- He, L.; Qin, S.; Liu, J.; Zhao, W.; Chang, T. Long-chain Brønsted acidic ionic liquids catalyzed one-pot three-component Biginelli reaction. World J. Eng. 2020, 17, 21–26. [Google Scholar] [CrossRef]
- Suzuki, I.; Suzumura, Y.; Takeda, K. Metal triflimide as a Lewis acid catalyst for Biginelli reactions in water. Tetrahedron Lett. 2006, 47, 7861–7864. [Google Scholar] [CrossRef] [Green Version]
- Kong, R.; Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Xie, Z.-B.; Gong, S.-S.; Sun, Q. Highly efficient synthesis of substituted 3, 4-Dihydropyrimidin-2-(1H)-ones (DHPMs) catalyzed by Hf(OTf)4: Mechanistic insights into reaction pathways under metal Lewis acid catalysis and solvent-free conditions. Molecules 2019, 24, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, L.M.; Ponce de Leon y Tobio, A.Y.; dos Santos, M.R.; de Oliveira, H.C.; Gomes, A.F.; Gozzo, F.C.; de Oliveira, A.L.; Neto, B.A. Mechanistic studies on lewis acid catalyzed biginelli reactions in ionic liquids: Evidence for the reactive intermediates and the role of the reagents. J. Org. Chem. 2012, 77, 10184–10193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Tang, G.M.; Wu, Y.S. A Set of phenyl sulfonate metal coordination complexes triggered Biginelli reaction for the high efficient synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones under solvent-free conditions. Appl. Organomet. Chem. 2020, 34, e5542. [Google Scholar] [CrossRef]
- Jetti, S.R.; Upadhyaya, A.; Jain, S. 3,4-Hydropyrimidin-2-(1H) one derivatives: Solid silica-based sulfonic acid catalyzed microwave-assisted synthesis and their biological evaluation as antihypertensive and calcium channel blocking agents. Med. Chem. Res. 2014, 23, 4356–4366. [Google Scholar] [CrossRef]
- Russowsky, D.; Canto, R.F.; Sanches, S.A.; D’Oca, M.G.; de Fátima, Â.; Pilli, R.A.; Kohn, L.K.; Antônio, M.A.; de Carvalho, J.E. Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: Monastrol, oxo-monastrol and oxygenated analogues. Bioorg. Chem. 2006, 34, 173–182. [Google Scholar] [CrossRef]
- Shumaila, A.M.; Al-Thulaia, A.A. Mini-review on the synthesis of Biginelli analogs using greener heterogeneous catalysis: Recent strategies with the support or direct catalyzing of inorganic catalysts. Synth. Commun. 2019, 49, 1613–1632. [Google Scholar] [CrossRef]
- Sheykhan, M.; Yahyazadeh, A.; Ramezani, L. A novel cooperative Lewis acid/Brønsted base catalyst Fe3O4@ SiO2-APTMS-Fe(OH)2: An efficient catalyst for the Biginelli reaction. Mol. Catal. 2017, 435, 166–173. [Google Scholar] [CrossRef]
- Narayanan, D.P.; Sankaran, S.; Narayanan, B.N. Novel rice husk ash-reduced graphene oxide nanocomposite catalysts for solvent free Biginelli reaction with a statistical approach for the optimization of reaction parameters. Mater. Chem. Phys. 2019, 222, 63–74. [Google Scholar] [CrossRef]
- Chopda, L.V.; Dave, P.N. Recent Advances in Homogeneous and Heterogeneous Catalyst in Biginelli Reaction from 2015-19: A Concise Review. ChemistrySelect 2020, 5, 5552–5572. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F. Pd immobilized on polymeric network containing imidazolium salt, cyclodextrin and carbon nanotubes: Efficient and recyclable catalyst for the hydrogenation of nitroarenes in aqueous media. J. Mol. Liq. 2020, 301, 112414. [Google Scholar] [CrossRef]
- Sadjadi, S. Halloysite-based hybrids/composites in catalysis. Appl. Clay Sci. 2020, 189, 105537. [Google Scholar] [CrossRef]
- Dehghani, S.; Sadjadi, S.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Study of the effect of the ligand structure on the catalytic activity of Pd@ ligand decorated halloysite: Combination of experimental and computational studies. Appl. Organomet. Chem. 2019, 33, e4891. [Google Scholar] [CrossRef]
- Sadjadi, S.; Lazzara, G.; Heravi, M.M.; Cavallaro, G. Pd supported on magnetic carbon coated halloysite as hydrogenation catalyst: Study of the contribution of carbon layer and magnetization to the catalytic activity. Appl. Clay Sci. 2019, 182, 105299. [Google Scholar] [CrossRef] [Green Version]
- Sadjadi, S.; Akbari, M.; Kahangi, F.G.; Heravi, M.M. Acidic polymer containing sulfunic acid and carboxylic acid groups heterogenized with natural clay: A novel metal free and heterogeneous catalyst for acid-catalyzed reactions. Polyhedron 2020, 179, 114375. [Google Scholar] [CrossRef]
- Yi, Q.; Qi, F.; Cheng, G.; Zhang, Y.; Xiao, B.; Hu, Z.; Liu, S.; Cai, H.; Xu, S. Thermogravimetric analysis of co-combustion of biomass and biochar. J. Therm. Anal. Calorim. 2013, 112, 1475–1479. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Mahdy, G.A.; Allohedan, H.A.; Abdullah, M.M. Synthesis and application of poly ionic liquid-based on 2-acrylamido-2-methyl propane sulfonic acid as corrosion protective film of steel. Int. J. Electrochem. Sci 2015, 10, 6106–6119. [Google Scholar]
- Morrison, D.W.; Forbes, D.C.; Davis, J.H. Base-promoted reactions in ionic liquid solvents. The Knoevenagel and Robinson annulation reactions. Tetrahedron Lett. 2001, 42, 6053–6055. [Google Scholar] [CrossRef]
- Javdannezhad, M.; Gorjizadeh, M.; Sayahi, M.H.; Sayyahi, S. Caffeine-loaded Fe3O4 nanoparticles: A new magnetically recoverable organocatalyst for Knoevenagel condensation reaction. J. Nanoanal. 2018, 5, 287–293. [Google Scholar]
- Das, A.; Anbu, N.; Dhakshinamoorthy, A.; Biswas, S. A highly catalytically active Hf (IV) metal-organic framework for Knoevenagel condensation. Microporous Mesoporous Mater. 2019, 284, 459–467. [Google Scholar] [CrossRef]
- Yue, C.; Mao, A.; Wei, Y.; Lü, M. Knoevenagel condensation reaction catalyzed by task-specific ionic liquid under solvent-free conditions. Catal. Commun. 2008, 9, 1571–1574. [Google Scholar] [CrossRef]
- Lolak, N.; Kuyuldar, E.; Burhan, H.; Goksu, H.; Akocak, S.; Sen, F. Composites of Palladium–Nickel Alloy Nanoparticles and Graphene Oxide for the Knoevenagel Condensation of Aldehydes with Malononitrile. ACS Omega 2019, 4, 6848–6853. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhou, S.; Kang, X.; Zhao, Y.; Yan, R.; Zhang, Y.; Wen, L. A Cationic Zinc–Organic Framework with Lewis Acidic and Basic Bifunctional Sites as an Efficient Solvent-Free Catalyst: CO2 Fixation and Knoevenagel Condensation Reaction. Inorg. Chem. 2018, 57, 11157–11164. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, W.; Liu, Z.; Yu, Y.; Dong, H.; Huo, P.; Yan, Y. Fabrication of the metal-free biochar-based graphitic carbon nitride for improved 2-Mercaptobenzothiazole degradation activity. J. Photochem. Photobiol. A 2018, 358, 284–293. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Zhang, Y.; Zou, W.; Luo, Y.; Dong, L.; Gao, B. Facile one-step synthesis of graphitic carbon nitride-modified biochar for the removal of reactive red 120 through adsorption and photocatalytic degradation. Biochar 2019, 1, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Freitas, E.F.; Souza, R.Y.; Passos, S.T.; Dias, J.A.; Dias, S.C.; Neto, B.A. Tuning the Biginelli reaction mechanism by the ionic liquid effect: The combined role of supported heteropolyacid derivatives and acidic strength. RSC Adv. 2019, 9, 27125–27135. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |









| NO. | Substrate | Yield (%) b |
|---|---|---|
| 1 | Benzaldehyde | 100 |
| 2 | 4-NO2-benzaldehyde | 98 |
| 3 | 2-NO2-benzaldehyde | 95 |
| 4 | 3-NO2-benzaldehyde | 95 |
| 5 | 4-Me-benzaldehyde | 98 |
| 6 | 4-MeO-benzaldehyde | 97 |
| 7 | 2-MeO-benzaldehyde | 95 |
| 8 | 4-Cl-benzaldehyde | 100 |
| 9 | Furfural | 90 |
| 10 | Acetophenone | 85 |
| Entry | Catalyst | Temp. °C | Solvent | Time (h:min) | Recycle Run | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Glycine | 25 | [6-mim]PF6 | 22:00 | 2 | 77 | [69] |
| 2 | Caffein-SiO2@Fe3O4 | 60 | H2O (ultrasonic irradiation) | 00:06 | 5 | 94 | [70] |
| 3 | Activated Hf-UiO-66-N2H3 | 25 | EtOH | 4:00 | 5 | 98 | [71] |
| 4 | [H3N+-CH2-CH2-OH][CH3COO−]IL | 25 | Solvent free | <1:00 | 5 | 90.9 | [72] |
| 5 | PdNi@GO | 25 | H2O/EtOH | 00:08 | 5 | 95 | [73] |
| 6 a | [Zn2(TCA)(BIB)2,5]·(NO3) | 60 | Solvent free | 1:00 | 4 | >99 | [74] |
| 7 | BC@GCN-P-IL | 25 | H2O | 2:00 | 5 | 100 | This work |

| NO. | Substrate | Yield (%) b |
|---|---|---|
| 1 | Benzaldehyde | 100 |
| 2 | 4-NO2-benzaldehyde | 98 |
| 3 | 2-NO2-benzaldehyde | 90 |
| 4 | 3-NO2-benzaldehyde | 93 |
| 5 | 4-Me-benzaldehyde | 95 |
| 6 | 4-MeO-benzaldehyde | 96 |
| 7 | 2-MeO-benzaldehyde | 90 |
| 8 | 4-Cl-benzaldehyde | 95 |
| Entry | Catalyst | Yield % |
|---|---|---|
| 1 | BC@GCN | 60 |
| 2 | BC@GCN-P | 85 |
| 3 | BC@GCN-IL | 90 |
| 4 | BC@GCN-P-IL | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadjadi, S.; Koohestani, F.; Heravi, M. Biochar-Based Graphitic Carbon Nitride Adorned with Ionic Liquid Containing Acidic Polymer: A Versatile, Non-Metallic Catalyst for Acid Catalyzed Reaction. Molecules 2020, 25, 5958. https://doi.org/10.3390/molecules25245958
Sadjadi S, Koohestani F, Heravi M. Biochar-Based Graphitic Carbon Nitride Adorned with Ionic Liquid Containing Acidic Polymer: A Versatile, Non-Metallic Catalyst for Acid Catalyzed Reaction. Molecules. 2020; 25(24):5958. https://doi.org/10.3390/molecules25245958
Chicago/Turabian StyleSadjadi, Samahe, Fatemeh Koohestani, and Majid Heravi. 2020. "Biochar-Based Graphitic Carbon Nitride Adorned with Ionic Liquid Containing Acidic Polymer: A Versatile, Non-Metallic Catalyst for Acid Catalyzed Reaction" Molecules 25, no. 24: 5958. https://doi.org/10.3390/molecules25245958
APA StyleSadjadi, S., Koohestani, F., & Heravi, M. (2020). Biochar-Based Graphitic Carbon Nitride Adorned with Ionic Liquid Containing Acidic Polymer: A Versatile, Non-Metallic Catalyst for Acid Catalyzed Reaction. Molecules, 25(24), 5958. https://doi.org/10.3390/molecules25245958