Discovering the Major Antitussive, Expectorant, and Anti-Inflammatory Bioactive Constituents in Tussilago farfara L. Based on the Spectrum–Effect Relationship Combined with Chemometrics
Abstract
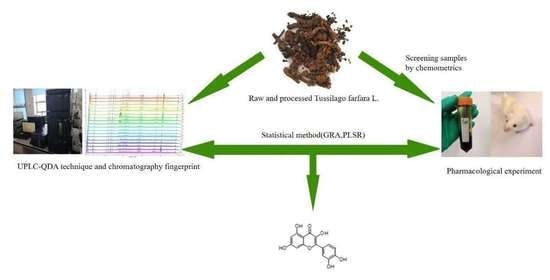
:1. Introduction
2. Results and Discussion
2.1. Results of UPLC Fingerprints
2.1.1. Optimization of UPLC Chromatographic Conditions
2.1.2. UPLC Fingerprints of FF Samples
2.1.3. SA of Fingerprints
2.1.4. Results of Hierarchical Cluster Analysis (HCA)
2.1.5. Results of PCA
2.1.6. Results of Screening Differences Samples
2.2. Results of Pharmacological Experiments
2.2.1. Results of Antitussive Experiments
2.2.2. Evaluation of Expectorant Experiments
2.2.3. Evaluation of Anti-Inflammatory Experiments
2.3. Analysis of Spectrum–Effect Relationships
2.3.1. Results of Grey Relational Analysis
2.3.2. Results of Partial Least Squares Regression Analysis
2.4. Discussion
3. Materials and Methods
3.1. Instruments
3.2. Materials and Reagents
3.3. Animals
3.4. UPLC-QDA Fingerprints
3.4.1. Establishment of UPLC Conditions
3.4.2. Preparation of Sample Solutions
3.4.3. Preparation of Standard Solutions
3.4.4. Method Validation of Fingerprint Analysis
3.4.5. Similarity Analysis of UPLC Fingerprints
3.4.6. PCA
3.4.7. HCA
3.5. Experiments of Pharmacodynamic Effects
3.5.1. Preparation of Gavage
3.5.2. Evaluation of Antitussive Activity
3.5.3. Evaluation of Expectorant Activity
3.5.4. Evaluation of Anti-Inflammatory Activity
3.6. The Spectrum-Effect Relationship
3.6.1. Grey Relational Analysis (GRA)
3.6.2. Partial Least Squares Regression (PLSR) Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farnsworth, N.R. Screening plants for new medicines. Biodiversity 1988, 15, 81–99. [Google Scholar]
- Jiang, Y.; David, B.; Tu, P.; Barbin, Y. Recent analytical approaches in quality control of traditional Chinese medicines—A review. Anal. Chim. Acta 2010, 657, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.S.; McDowell, A.; Strachan, C.J.; Gordon, K.C. Evaluation of vibrational spectroscopic methods to identify and quantify multiple adulterants in herbal medicines. Talanta 2015, 138, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Sun, Y.; Liu, S.; Song, F.; Pi, Z.; Liu, Z. Stepwise targeted matching strategy from in vitro to in vivo based on ultra–high performance liquid chromatography tandem mass spectrometry technology to quickly identify and screen pharmacodynamic constituents. Talanta 2019, 194, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tistaert, C.; Thierry, L.; Szandrach, A.; Dejaegher, B.; Fan, G.; Frédérich, M.; Vander Heyden, Y. Quality control of Citri reticulatae pericarpium: Exploratory analysis and discrimination. Anal. Chim. Acta 2011, 705, 111–122. [Google Scholar] [CrossRef]
- Liang, X.M.; Jin, Y.; Wang, Y.P.; Jin, G.W.; Fu, Q.; Xiao, Y.S. Qualitative and quantitative analysis in quality control of traditional Chinese medicines. J. Chromatogr. A 2009, 1216, 2033–2044. [Google Scholar] [CrossRef]
- Xie, P.S.; Leung, A.Y. Understanding the traditional aspect of Chinese medicine in order to achieve meaningful quality control of Chinese materia medica. J. Chromatogr. A 2009, 1216, 1933–1940. [Google Scholar] [CrossRef]
- Xu, C.J.; Liang, Y.Z.; Chau, F.T.; Heyden, Y.V. Pretreatments of chromatographic fingerprints for quality control of herbal medicines. J. Chromatogr. A 2006, 1134, 253–259. [Google Scholar] [CrossRef]
- Xu, G.L.; Xie, M.; Yang, X.Y.; Song, Y.; Yan, C.; Yang, Y.; Zhang, X.; Liu, Z.Z.; Tian, Y.X.; Wang, Y.; et al. Spectrum-effect relationships as a systematic approach to traditional chinese medicine research: Current status and future perspectives. Molecules 2014, 19, 17897–17925. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, C.; Gong, X.; Sun, X.; Li, H.; Zhao, Y.; Zhou, X. Quantification and Efficient Discovery of Quality Control Markers for Emilia prenanthoidea DC. by fingerprint-efficacy relationship modelling. J. Pharm. Biomed. Anal. 2018, 156, 36–44. [Google Scholar]
- Wang, F.; Xiong, Z.Y.; Li, P.; Yang, H.; Gao, W.; Li, H.J. From chemical consistency to effective consistency in precise quality discrimination of Sophora flower-bud and Sophora flower: Discovering efficacy-associated markers by fingerprint-activity relationship modeling. J. Pharm. Biomed. Anal. 2017, 132, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Chen, J.; Yang, J.L.; Shi, Y.P. UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta 2018, 180, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Lin, Z.J.; Xiao, M.L.; Niu, H.J.; Zhang, B. The spectrum-effect relationship-a rational approach to screening effective compounds, reflecting the internal quality of Chinese herbal medicine. Chin. J. Nat. Med. 2016, 14, 177–184. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhu, T.; Zhu, H.; Zhu, X.; Cai, H.; Cao, G.; Xu, X.; Niu, M.; Cai, B. Study on spectrum-effect correlation for screening the effective components in Fangji Huangqi Tang basing on ultra-high performance liquid chromatography-mass spectrometry. Phytomedicine 2018, 47, 81–92. [Google Scholar] [CrossRef]
- Wang, J.; Luo, D.; Liang, M.; Zhang, T.; Yin, X.; Zhang, Y.; Yang, X.; Liu, W. Spectrum-Effect Relationships between High-Performance Liquid Chromatography (HPLC) Fingerprints and the Antioxidant and Anti-Inflammatory Activities of Collagen Peptides. Molecules 2018, 23, 3257. [Google Scholar] [CrossRef] [Green Version]
- Segheto, L.; Santos, B.C.S.; Werneck, A.F.L.; Vilela, F.M.P.; de Sousa, O.V.; Rodarte, M.P. Antioxidant extracts of coffee leaves and its active ingredient 5-caffeoylquinic acid reduce chemically-induced inflammation in mice. Ind. Crop. Prod. 2018, 126, 48–57. [Google Scholar] [CrossRef]
- Wu, Q.Z.; Zhao, D.X.; Xiang, J.; Zhang, M.; Zhang, C.F.; Xu, X.H. Antitussive, expectorant, and anti-inflammatory activities of four caffeoylquinic acids isolated from Tussilago farfara. Pharm. Biol. 2016, 54, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zhang, F.; Qin, Q.; Shang, Y.; Wan, D. In Vivo Evaluation of the Antiasthmatic, Antitussive, and Expectorant Activities and Chemical Components of Three Elaeagnus Leaves. Evid. Based Complement. Altern. Med. 2015, 2015, 1–7. [Google Scholar]
- Cheon, H.J.; Nam, S.H.; Kim, J.K. Tussilagone, a major active component in Tussilago farfara, ameliorates inflammatory responses in dextran sulphate sodium-induced murine colitis. Chem. -Biol. Interact. 2018, 294, 74–80. [Google Scholar] [CrossRef]
- Yang, A.; Shang, Q.; Yang, L.; Li, C.; Yuan, H.J. Chemical Constituents of the Flower Buds of Tussilago farfara. II. Chem. Nat. Compd. 2018, 54, 978–980. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, H.; Xing, X.; Yan, M.; Guo, X.; Man, W.; Hou, A.; Yang, L. A Biosensor-Based Quantitative Analysis System of Major Active Ingredients in Lonicera japonica Thunb. Using UPLC-QDa and Chemometric Analysis. Molecules 2019, 24, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.J.; Jiang, Z.M.; Xiao, P.T.; Sun, J.B.; Bi, Z.M.; Liu, E.H. Identification of anti-inflammatory components in Sinomenii Caulis based on spectrum-effect relationship and chemometric methods. J. Pharm. Biomed. Anal. 2019, 167, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-L.; Wang, M.; Ren, X. Application progress on chemical pattern recognition in quality control of Chinese materia medica. Chin. Tradit. Herb. Drugs 2017, 48, 4339–4345. [Google Scholar]
- Tang, Q.-Y.; Zhang, C.-X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |






| Peak | Compound | Retention Time (Min) | Precursor (m/z) | Adduct | Structure |
|---|---|---|---|---|---|
| P1 | 3-CQA (Chlorogenic acid) | 3.47 | 376.98 | [M + Na]+ |  |
| P2 | Unknown | 10.48 | 366.35 | Unknown | Unknown |
| P3 | Rutin | 11.33 | 633.15 | [M + Na]+ |  |
| P4 | Unknown | 11.56 | 487.28 | Unknown | Unknown |
| P5 | 3,5-CQA (3,5-Dicaffeoylquinic acid) | 12.64 | 539.21 | [M + Na]+ |  |
| P6 | 3,4-CQA (3,4-Dicaffeoylquinic acid) | 12.98 | 538.92 | [M + Na]+ |  |
| P7 | 4,5-CQA (4,5-Dicaffeoylquinic acid) | 14.04 | 538.99 | [M + Na]+ |  |
| P8 | Kaempferol | 17.97 | 286.90 | [M + H]+ |  |
| P9 | Unknown | 27.10 | 453.21 | Unknown | Unknown |
| P10 | Unknown | 27.73 | 413.15 | Unknown | Unknown |
| P11 | Unknown | 28.58 | 471.08 | Unknown | Unknown |
| P12 | Unknown | 36.94 | 453.14 | Unknown | Unknown |
| P13 | Unknown | 39.82 | 513.32 | Unknown | Unknown |
| P14 | Unknown | 47.60 | 453.36 | Unknown | Unknown |
| Peak No. | RRT | R.S.D. (%) | RPA | R.S.D. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Precision | Repeatability | Stability | Precision | Repeatability | Stability | |||
| 1 | 1.000 | 0.19 | 1.14 | 0.92 | 1.000 | 3.55 | 2.85 | 3.95 |
| 2 | 1.094 | 0.34 | 0.28 | 0.99 | 0.268 | 3.85 | 3.61 | 2.90 |
| 3 | 1.193 | 0.33 | 0.64 | 0.61 | 0.918 | 3.53 | 3.64 | 4.23 |
| 4 | 1.216 | 0.53 | 0.71 | 0.55 | 1.088 | 4.32 | 3.01 | 2.91 |
| 5 | 1.306 | 0.28 | 1.62 | 0.86 | 0.776 | 3.74 | 3.55 | 4.46 |
| 6 | 1.668 | 1.13 | 0.52 | 0.92 | 0.825 | 4.33 | 3.05 | 5.02 |
| 7 | 1.752 | 0.74 | 0.46 | 0.91 | 0.086 | 4.76 | 3.92 | 4.46 |
| 8 | 1.816 | 0.68 | 0.89 | 0.51 | 0.152 | 4.28 | 3.60 | 4.08 |
| 9 | 2.511 | 0.44 | 0.67 | 0.87 | 0.535 | 2.53 | 2.60 | 3.73 |
| 10 | 2.593 | 0.57 | 1.33 | 1.03 | 2.100 | 2.44 | 3.36 | 3.77 |
| 11 | 2.671 | 0.60 | 0.73 | 0.88 | 2.132 | 2.04 | 3.11 | 3.92 |
| 12 | 3.353 | 1.05 | 1.11 | 0.82 | 1.744 | 4.41 | 4.62 | 3.92 |
| 13 | 3.654 | 0.94 | 0.83 | 0.69 | 6.995 | 3.81 | 2.76 | 2.55 |
| 14 | 4.358 | 0.45 | 0.37 | 1.02 | 0.922 | 2.48 | 2.60 | 4.48 |
| NO. | SA | NO. | SA |
|---|---|---|---|
| M1 | 0.98 | S10 | 0.91 |
| M2 | 0.90 | S11 | 0.79 |
| M3 | 0.92 | S12 | 0.98 |
| M4 | 0.94 | S13 | 0.92 |
| M5 | 0.90 | S14 | 0.87 |
| M6 | 0.93 | S15 | 0.94 |
| M7 | 0.92 | S16 | 0.74 |
| S8 | 0.93 | S17 | 0.85 |
| S9 | 0.92 | S18 | 0.90 |
| Previous Number | Renumber | Previous Number | Renumber |
|---|---|---|---|
| S8 | X1 | S15 | X6 |
| S9 | X2 | M1 | Y1 |
| S10 | X3 | M3 | Y2 |
| S12 | X4 | M5 | Y3 |
| S14 | X5 | M6 | Y4 |
| Antitussive Effect | Expectorant Effect | Anti-Inflammatory Effect | ||||
|---|---|---|---|---|---|---|
| Order | Peak | Correlation Coefficient | Peak | Correlation Coefficient | Peak | Correlation Coefficient |
| 1 | P5 | 0.6945 | P14 | 0.7648 | P14 | 0.7307 |
| 2 | P8 | 0.6850 | P9 | 0.7481 | P9 | 0.6844 |
| 3 | P12 | 0.6821 | P12 | 0.7361 | P2 | 0.6506 |
| 4 | P7 | 0.6818 | P10 | 0.7315 | P13 | 0.6495 |
| 5 | P14 | 0.6807 | P11 | 0.7267 | P10 | 0.6482 |
| 6 | P10 | 0.6645 | P4 | 0.7261 | P11 | 0.6426 |
| 7 | P9 | 0.6644 | P3 | 0.7130 | P12 | 0.6393 |
| 8 | P3 | 0.6611 | P2 | 0.7044 | P3 | 0.6386 |
| 9 | P4 | 0.6557 | P7 | 0.7014 | P4 | 0.6364 |
| 10 | P11 | 0.6473 | P13 | 0.6919 | P1 | 0.6337 |
| 11 | P13 | 0.6408 | P6 | 0.6868 | P8 | 0.6337 |
| 12 | P6 | 0.6380 | P1 | 0.6791 | P6 | 0.6254 |
| 13 | P2 | 0.6273 | P5 | 0.6748 | P5 | 0.6228 |
| 14 | P1 | 0.6234 | P8 | 0.6550 | P7 | 0.6184 |
| NO. | Origin | NO. | Origin |
|---|---|---|---|
| M1 | Yunnan | S10 | Gansu |
| M2 | Gansu | S11 | Hubei |
| M3 | Sichuan | S12 | Hubei |
| M4 | Jiangxi | S13 | Hubei |
| M5 | Heilongjiang | S14 | Gansu |
| M6 | Hebei | S15 | Gansu |
| M7 | Hebei | S16 | Hebei |
| S8 | Anhui | S17 | Heilongjiang |
| S9 | Anhui | S18 | Anhui |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Jiang, H.; Wang, S.; Hou, A.; Man, W.; Zhang, J.; Guo, X.; Yang, B.; Kuang, H.; Wang, Q. Discovering the Major Antitussive, Expectorant, and Anti-Inflammatory Bioactive Constituents in Tussilago farfara L. Based on the Spectrum–Effect Relationship Combined with Chemometrics. Molecules 2020, 25, 620. https://doi.org/10.3390/molecules25030620
Yang L, Jiang H, Wang S, Hou A, Man W, Zhang J, Guo X, Yang B, Kuang H, Wang Q. Discovering the Major Antitussive, Expectorant, and Anti-Inflammatory Bioactive Constituents in Tussilago farfara L. Based on the Spectrum–Effect Relationship Combined with Chemometrics. Molecules. 2020; 25(3):620. https://doi.org/10.3390/molecules25030620
Chicago/Turabian StyleYang, Liu, Hai Jiang, Song Wang, Ajiao Hou, Wenjing Man, Jiaxu Zhang, Xinyue Guo, Bingyou Yang, Haixue Kuang, and Qiuhong Wang. 2020. "Discovering the Major Antitussive, Expectorant, and Anti-Inflammatory Bioactive Constituents in Tussilago farfara L. Based on the Spectrum–Effect Relationship Combined with Chemometrics" Molecules 25, no. 3: 620. https://doi.org/10.3390/molecules25030620
APA StyleYang, L., Jiang, H., Wang, S., Hou, A., Man, W., Zhang, J., Guo, X., Yang, B., Kuang, H., & Wang, Q. (2020). Discovering the Major Antitussive, Expectorant, and Anti-Inflammatory Bioactive Constituents in Tussilago farfara L. Based on the Spectrum–Effect Relationship Combined with Chemometrics. Molecules, 25(3), 620. https://doi.org/10.3390/molecules25030620