First Cobalt(II) Spin Crossover Compound with N4S2-Donorset
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis
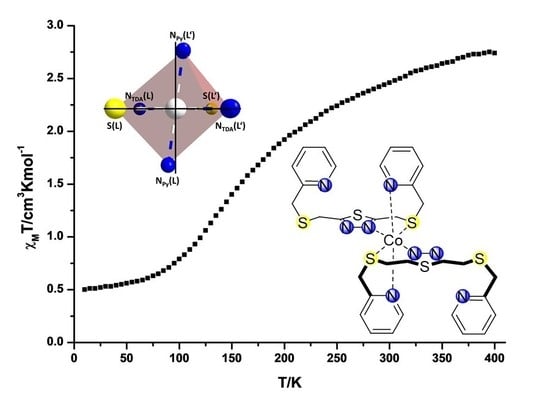
2.2. Variable Temperature Magnetic Susceptibility Measurements
2.3. Crystal Structures
3. Materials and Methods
3.1. General Methods and Materials
3.2. Ligand Synthesis
2,5-Bis[(2-pyridylmethyl)thio]methyl-1,3,4-thiadiazole (L)
3.3. Complex Synthesis
3.3.1. [FeII(L)2](ClO4)2 (C1)
3.3.2. [CoII(L)2](ClO4)2 (C2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, ISBN 978-3-540-40394-4. [Google Scholar]
- Gütlich, P.; Goodwin, H.A. Spin Crossover in Transition Metal Compounds II; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 234, ISBN 978-3-540-40396-8. [Google Scholar]
- Gütlich, P.; Goodwin, H.A. Spin Crossover in Transition Metal Compounds III; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; Volume 235, ISBN 978-3-540-40395-1. [Google Scholar]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials; John Wiley & Sons Ltd.: Oxford, UK, 2013; ISBN 9781118519301. [Google Scholar]
- Kahn, O. Spin-Transition Polymers: From Molecular Materials toward Memory Devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermisch und optisch schaltbare Eisen(II)-Komplexe. Angew. Chem. 1994, 106, 2109–2141. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Brooker, S. A toolbox of building blocks, linkers and crystallisation methods used to generate single-chain magnets. Coord. Chem. Rev. 2015, 296, 24–44. [Google Scholar] [CrossRef]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [Green Version]
- Barrios, L.A.; Peyrecave-Lleixà, E.; Craig, G.A.; Roubeau, O.; Teat, S.J.; Aromí, G. Unusual Crystal Packing in a Family of [Fe{2,6-bis(pyrazol-3-yl)pyridine} 2 ] 2+ Compounds and the Effect on the Occurrence of Spin Crossover and Its Cooperative Character. Eur. J. Inorg. Chem. 2014, 2014, 6013–6021. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef]
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; Volume 1, pp. 1–47. [Google Scholar]
- Roberts, T.D.; Little, M.A.; Kershaw Cook, L.J.; Halcrow, M.A. Iron(ii) complexes of 2,6-di(1H-pyrazol-3-yl)-pyridine derivatives with hydrogen bonding and sterically bulky substituents. Dalt. Trans. 2014, 43, 7577–7588. [Google Scholar] [CrossRef]
- Bauer, W.; Lochenie, C.; Weber, B. Synthesis and characterization of 1D iron( ii ) spin crossover coordination polymers with hysteresis. Dalt. Trans. 2014, 43, 1990–1999. [Google Scholar] [CrossRef]
- Kahn, O.; Kröber, J.; Jay, C. Spin Transition Molecular Materials for displays and data recording. Adv. Mater. 1992, 4, 718–728. [Google Scholar] [CrossRef]
- Kröber, J.; Codjovi, E.; Kahn, O.; Grolière, F.; Jay, C. A Spin Transition System with a Thermal Hysteresis at Room Temperature. J. Am. Chem. Soc. 1993, 115, 9810–9811. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Niel, V.; Muñoz, M.C. Communication between iron(II) building blocks in cooperative spin transition phenomena. Coord. Chem. Rev. 2003, 236, 121–141. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalt. Trans. 2005, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Rentschler, E. The First 1,3,4-Oxadiazole Based Dinuclear Iron(II) Complexes Showing Spin Crossover Behavior with Hysteresis. Eur. J. Inorg. Chem. 2016, 2016, 1955–1960. [Google Scholar] [CrossRef]
- Herold, C.F.; Carrella, L.M.; Rentschler, E. A Family of Dinuclear Iron(II) SCO Compounds Based on a 1,3,4-Thiadiazole Bridging Ligand. Eur. J. Inorg. Chem. 2015, 2015, 3632–3636. [Google Scholar] [CrossRef]
- Fürmeyer, F.; Carrella, L.M.; Ksenofontov, V.; Möller, A.; Rentschler, E. Phase trapping in multistep spin crossover compound. Inorg. Chem. 2020, in press. [Google Scholar]
- Hogue, R.W.; Feltham, H.L.C.; Miller, R.G.; Brooker, S. Spin Crossover in Dinuclear N 4 S 2 Iron(II) Thioether–Triazole Complexes: Access to [HS-HS], [HS-LS], and [LS-LS] States. Inorg. Chem. 2016, 55, 4152–4165. [Google Scholar] [CrossRef]
- Hayami, S.; Komatsu, Y.; Shimizu, T.; Kamihata, H.; Lee, Y.H. Spin-crossover in cobalt(II) compounds containing terpyridine and its derivatives. Coord. Chem. Rev. 2011, 255, 1981–1990. [Google Scholar] [CrossRef]
- Grillo, A.V.; Gahan, R.L.; Hanson, R.G.; Stranger, R.; Hambley, W.T.; Murray, S.K.; Moubaraki, B.; Cashion, D.J. Iron(III) and iron(II) complexes of 1-thia-4{,}7-diazacyclononane ([9]aneN2S) and 1{,}4-dithia-7-azacyclononane ([9]aneNS2). X-Ray structural analyses{,} magnetic susceptibility{,} Mössbauer{,} EPR and electronic spectroscopy †. J. Chem. Soc., Dalt. Trans. 1998, 2341–2348. [Google Scholar] [CrossRef]
- England, J.; Gondhia, R.; Bigorra-Lopez, L.; Petersen, A.R.; White, A.J.P.; Britovsek, G.J.P. Towards robust alkane oxidation catalysts: Electronic variations in non-heme iron(ii) complexes and their effect in catalytic alkane oxidation. Dalt. Trans. 2009, 27, 5319–5334. [Google Scholar] [CrossRef] [Green Version]
- Reus, C.; Ruth, K.; Tüllmann, S.; Bolte, M.; Lerner, H.-W.; Weber, B.; Holthausen, M.C.; Wagner, M. Synthesis, Molecular Structure, and Physical Properties of the Complexes [{PhB(pz)2(CH2SMe)}2M] (M = MnII, FeII; pz = pyrazol-1-yl) Containing a Novel [N,N,S]-Heteroscorpionate Ligand. Eur. J. Inorg. Chem. 2011, 2011, 1709–1718. [Google Scholar] [CrossRef]
- Lennartson, A.; Bond, A.D.; Piligkos, S.; McKenzie, C.J. Four-Site Cooperative Spin Crossover in a Mononuclear Fe II Complex. Angew. Chem. 2012, 124, 11211–11214. [Google Scholar] [CrossRef]
- Lennartson, A.; Southon, P.; Sciortino, N.F.; Kepert, C.J.; Frandsen, C.; Mørup, S.; Piligkos, S.; McKenzie, C.J. Reversible Guest Binding in a Non-Porous Fe II Coordination Polymer Host Toggles Spin Crossover. Chem.-A Eur. J. 2015, 21, 16066–16072. [Google Scholar] [CrossRef]
- Arroyave, A.; Lennartson, A.; Dragulescu-Andrasi, A.; Pedersen, K.S.; Piligkos, S.; Stoian, S.A.; Greer, S.M.; Pak, C.; Hietsoi, O.; Phan, H.; et al. Spin Crossover in Fe(II) Complexes with N 4 S 2 Coordination. Inorg. Chem. 2016, 55, 5904–5913. [Google Scholar] [CrossRef]
- Yergeshbayeva, S.; Hrudka, J.J.; Lengyel, J.; Erkasov, R.; Stoian, S.A.; Dragulescu-Andrasi, A.; Shatruk, M. Heteroleptic Fe(II) Complexes with N 4 S 2 Coordination as a Platform for Designing Spin-Crossover Materials. Inorg. Chem. 2017, 56, 11096–11103. [Google Scholar] [CrossRef]
- Wei, Z.; Xie, X.; Zhao, J.; Huang, L.; Liu, X. A novel hexadentate ligand and its complexes with divalent metal ions (Zinc, Copper, and Cobalt): Synthesis, characterization, and electrochemical investigation. Inorg. Chim. Acta 2012, 387, 277–282. [Google Scholar] [CrossRef]
- Funkemeier, D.; Mattes, R. Synthesis and structural studies of copper(II), nickel(II) and cobalt(II) complexes of a 14-membered trans-N2S2 dibenzo macrocycle with two pendant pyridylmethyl groups. J. Chem. Soc. Dalton Trans. 1993, 1313–1319. [Google Scholar] [CrossRef]
- Mohamadou, A.; Jubert, C.; Barbier, J.-P. Novel cobalt(II) complexes with pyridyl/ether or pyridyl/thioether ligands. The conversion of pyridyl/thioether cobalt(II) complex to pyridyl/sulfinato cobalt(III) compound. Inorg. Chim. Acta 2006, 359, 273–282. [Google Scholar] [CrossRef]
- Magwa, N.P.; Hosten, E.; Watkins, G.M.; Tshentu, Z.R. The coordination and extractive chemistry of the later 3d transition metals with bis ((1 R -benzimidazol-2-yl)methyl)sulfide. J. Coord. Chem. 2013, 66, 114–125. [Google Scholar] [CrossRef]
- Hogue, R.W.; Schott, O.; Hanan, G.S.; Brooker, S. A Smorgasbord of 17 Cobalt Complexes Active for Photocatalytic Hydrogen Evolution. Chem. A Eur. J. 2018, 24, 9820–9832. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Z.; Wang, F.; Peng, H.; Zhang, H.; Wang, C.; Wang, K. V-shaped ligand 1,3-bis(1-ethylbenzimidazol-2-yl)-2-thiapropane and manganese(II), cobalt(II) and copper(II) complexes: Synthesis, crystal structure, DNA-binding properties and antioxidant activities. J. Photochem. Photobiol. B Biol. 2015, 148, 252–261. [Google Scholar] [CrossRef]
- Pandiyan, T.; Hernández, J.G.; Medina, N.T.; Bernés, S. Geometrical isomers of bis(benzimidazol-2-ylethyl)sulfide)cobalt(II) diperchlorates: Synthesis, structure, spectra and redox behavior of pink-[Co(bbes)2](ClO4)2 and blue-[Co(bbes)2](ClO4)2. Inorg. Chim. Acta 2004, 357, 2570–2578. [Google Scholar] [CrossRef]
- Singh, A.K.; Mukherjee, R. Cobalt(ii) and cobalt(iii) complexes of thioether-containing hexadentate pyrazine amide ligands: C–S bond cleavage and cyclometallation reaction. J. Chem. Soc. Dalt. Trans. 2008, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Bannerjee, D.; Datta, P.; Lu, T.H.; Slawin, A.M.Z.; Sinha, C. Cobalt-thioalkylazoimidazole complexes: Structures, spectra and redox properties. Polyhedron 2009, 28, 3519–3525. [Google Scholar] [CrossRef]
- Thomas, L.; Hsiung, T.; Breen, J.; Worrell, J.H. Kinetics of substitution and isomerization of nitrite ion on aqua(7-methyl-4, 10-dithia-1, 7, 13- triazatridecane)cobalt(III) and the structure of the product. J. Coord. Chem. 1992, 26, 15–34. [Google Scholar]
- Eaborn, C. Purification of Laboratory Chemicals. J. Organomet. Chem. 1981. [Google Scholar] [CrossRef]
- Cobas, J.C.; Sardina, F.J. Nuclear magnetic resonance data processing. MestRe-C: A software package for desktop computers. Concepts Magn. Reson. 2003, 19A, 80–96. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Not available. |






| Selected Parameters [a] | C1 (@173 K) | C2 (@120 K) | C2 (@250 K) |
|---|---|---|---|
| M-NTDA(L) | 1.967(1) | 1.979(4) | 2.057(2) |
| M-NPy(L) | 2.014(1) | 2.019(4) | 2.075(2) |
| M-NTDA(L′) | 1.964(1) | 1.981(4) | |
| M-NPy(L′) | 2.016(1) | 2.027(4) | |
| M-S(L) | 2.263(1) | 2.472(2) | 2.479(1) |
| M-S(L′) | 2.264(1) | 2.470(2) | |
| cis NTDA-M-NPy | 84.1–95.9 | 84.6–95.6 | 85.1, 94.1 |
| cis NTDA-M-S | 85.3–94.8 | 82.9–97.4 | 83.8, 96.2 |
| cis NPy-M-S | 84.8–95.2 | 83.2–96.7 | 82.8, 97.2 |
| av. trans X-M-X | 179.8 | 179.6 | 180.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fürmeyer, F.; Münzberg, D.; Carrella, L.M.; Rentschler, E. First Cobalt(II) Spin Crossover Compound with N4S2-Donorset. Molecules 2020, 25, 855. https://doi.org/10.3390/molecules25040855
Fürmeyer F, Münzberg D, Carrella LM, Rentschler E. First Cobalt(II) Spin Crossover Compound with N4S2-Donorset. Molecules. 2020; 25(4):855. https://doi.org/10.3390/molecules25040855
Chicago/Turabian StyleFürmeyer, Fabian, Danny Münzberg, Luca M. Carrella, and Eva Rentschler. 2020. "First Cobalt(II) Spin Crossover Compound with N4S2-Donorset" Molecules 25, no. 4: 855. https://doi.org/10.3390/molecules25040855
APA StyleFürmeyer, F., Münzberg, D., Carrella, L. M., & Rentschler, E. (2020). First Cobalt(II) Spin Crossover Compound with N4S2-Donorset. Molecules, 25(4), 855. https://doi.org/10.3390/molecules25040855