Insecticidal and Biting Deterrent Activities of Magnolia grandiflora Essential Oils and Selected Pure Compounds against Aedes aegypti
Abstract
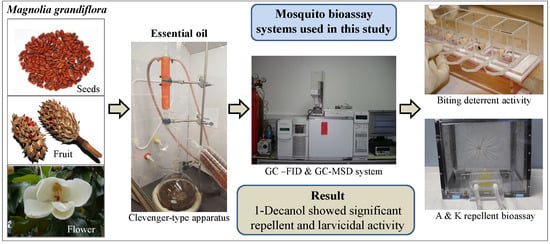
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Extraction of Essential Oils
4.4. GC-MS Analysis
4.5. GC Analysis
4.6. Insects
4.7. Mosquito Biting Bioassay
4.8. In vitro A & K Repellent Bioassay
4.9. Larval Bioassay
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Abbas, A.; Debboun, M. Zika virus: Epidemiology, vector and sexual transmission neurological disorders and vector management—A review. Int. J. Curr. Res. 2017, 10, 58721–58737. [Google Scholar]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Leal, W.S. The enigmatic reception of DEET—The gold standard of insect repellents. Currt. Opin. Insect Sci. 2006, 6, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Frances, S.P. Efficacy and safety of repellents containing DEET. In Insect Repellents: Principles, Methods and Uses; Debboun, M., Frances, S.P., Stickman, D., Eds.; CRC Press: New York, NY, USA, 2007; pp. 311–325. [Google Scholar]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhan, J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2006, 57, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Avonto, C.; Wang, M.; Parcher, J.F.; Ali, A.; Demirci, B.; Raman, V.; Khan, I.A. Comparative investigation of Umbellularia californica and Laurus nobilis leaf essential oils and identification of constituents active against Aedes aegypti. J. Agric Food Chem. 2013, 61, 12283–12291. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Baser, K.H.C.; Khan, I.A. Chemical composition and biological activity of essential oils from four Nepeta species and hybrids against Aedes aegypti (L.) (Diptera: Culicidae). Rec. Nat. Prod. 2016, 10, 137–147. [Google Scholar]
- Cantrell, C.L.; Maxwell, A.P.; Ali, A. Isolation and identification of mosquito (Aedes aegypti) biting-deterrent compounds from the native American ethnobotanical remedy plant Hierochloe odorata (Sweetgrass). J. Agric. Food Chem. 2016, 63, 447–456. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, R.; Wang, X.; Liu, S.; Fan, Z. Chemical composition of volatile constituents of Magnolia grandiflora. Chem. Nat. Compounds 2009, 45, 257–258. [Google Scholar] [CrossRef]
- Rao, K.V.; Wu, W.N. Glycosides of Magnolia grandiflora. III: Structural elucidation of magnolenin C. J. Nat. Prod. 1978, 41, 56–62. [Google Scholar]
- El-Feraly, F.S. Melampolides from Magnolia grandiflora. Phytochemistry 1984, 23, 2372–2374. [Google Scholar] [CrossRef]
- Rao, K.V.; Davis, T.L. Constituents of Magnolia grandiflora. III. Toxic principle of the wood. J. Nat. Prod. 1982, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Schuhly, W.; Ross, S.A.; Mehmedic, Z.; Fischer, N.H. Essential oils of the follicles of four North American Magnolia Species. Nat. Prod. Commun. 2008, 3, 1117–1119. [Google Scholar] [CrossRef] [Green Version]
- Guerra-Boone, L.; Alvarez-Román, R.; Salazar-Aranda, R.; Torres-Cirio, A.; Rivas-Galindo, V.M.; Waksman de Torres, N.; González González, G.M.; Pérez-López, L.A. Chemical compositions and antimicrobial and antioxidant activities of the essential oils from Magnolia grandiflora, Chrysactinia mexicana, and Schinus molle found in northeast Mexico. Nat. Prod. Commun. 2013, 8, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.N.; Kumar, S. Volatile constituents from the flowers of Magnolia grandiflora L. From Lucknow, India. J. Essent. Oils Res. 1999, 11, 633–634. [Google Scholar] [CrossRef]
- Farag, M.A.; Al-Mahdy, D.A. Comparative study of the chemical composition and biological activities of Magnolia grandiflora and Magnolia virginiana flower essential oils. Nat. Prod. Res. 2013, 27, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.R.R.; Sastry, K.P.; Saleem, S.M.; Rao, E.V.S.P.; Syamasundar, K.V.; Ramesh, S. Volatile flower oils of three genotypes of rose-scented geranium (Pelargonium sp.). Flavour Fragr. J. 2000, 15, 105–107. [Google Scholar]
- Ali, A.; Tabanca, N.; Demirci, B.; Baser, K.H.C.; Ellis, J.; Gray, S.; Lackey, B.R.; Murphy, C.; Khan, I.A.; Wedge, D.E. Composition, mosquito larvicidal, biting deterrent and antifungal activity of essential oils of different plant parts of Cupressus arizonica var. glabra (Sudw.) Little (‘Carolina Sapphire’). Nat. Prod. Commun. 2013, 8, 257–260. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Kurkcuoglu, M.; Duran, A.; Blyhthe, E.K.; Khan, I.A.; Baser, K.H.C. Chemical composition, larvicidal and biting deterrent activity of essential oils of two subspecies of Tanacetum argenteum (Lam.) Willd. and individual constituents against Aedes aegypti (L.) (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 824–830. [Google Scholar] [CrossRef]
- Tabanca, N.; Gao, Z.; Demirci, B.; Techen, N.; Wedge, D.E.; Ali, A.; Sampson, B.J.; Werle, C.; Bernier, U.R.; Khan, I.A.; et al. Molecular and phytochemical investigation of Angelica dahurica and A. pubescens essential oils and their biological activity against Aedes aegypti, Stephanitis pyrioides and Colletotrichum Species. J. Agric. Food Chem. 2014, 62, 8848–8857. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential oils of Echinophora lamondiana (Apiales: Umbelliferae): A relationship between chemical profile and biting deterrence and larvicidal activity against mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 93–100. [Google Scholar] [CrossRef]
- Ali, A.; Cantrell, C.L.; Bernier, U.R.; Duke, S.O.; Schneider, J.C.; Khan, I. Aedes aegypti (Diptera: Culicidae) Biting deterrence: Structure-activity relationship of saturated and unsaturated fatty acids. J. Med. Entomol. 2012, 49, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Bernier, U.R.; Ali, A.; Wang, M.; Demirci, B.; Blythe, E.K.; Khan, S.I.; Baser, K.H.C.; Khan, I.A. Bioassay-guided investigation of two Monarda essential oils for repellent activity against yellow fever mosquito Aedes aegypti. J. Agric. Food Chem. 2013, 61, 8573–8580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Estevez, N.; Vasquez-Morales, S.G.; Cano-Medina, T.; Sanchez-Velasquez, L.R.; Noa-Carrazana, J.C.; Diaz-Fleischer, F. Insecticidal activity of raw ethanolic extracts from Magnolia dealbata Zucc. on a tephritid pest. J. Environ. Sci. Health B 2013, 48, 582–586. [Google Scholar] [CrossRef] [PubMed]
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; J. Wiley and Sons: New York, NY, USA, 1989. [Google Scholar]
- Hochmuth, D.H. MassFinder 4.0, Hochmuth Scientific Consulting, Hamburg, Germany, 2008.
- Klun, J.A.; Kramer, M.; Debboun, M. A new in vitro bioassay system for discovery of novel human-use mosquito repellents. J. Am. Mosq. Cont. Assoc. 2005, 21, 64–70. [Google Scholar] [CrossRef]
- Ali, A.; Cantrell, C.L.; Khan, I.A. A new in vitro bioassay system for the discovery and quantitative evaluation of mosquito repellents. J. Med. Entomol. 2017, 54, 1328–1336. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Wang, W.; Slavov, S.; Dobchev, D.A.; Hall, C.D.; Tsikolia, M.; Bernier, U.R.; Elejalde, N.M.; Clark, G.G.; Linthicum, K.J. Novel carboxamides as potential mosquito repellents. J. Med. Entomol. 2010, 47, 924–938. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. A high-throughput screening method to identify potential pesticides for mosquito control. J. Med. Entomol. 2009, 46, 335–341. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute. SAS Online Doc., version 9.2; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds 1-decanol, 1-octanol, and 1-heptanol. are available from A.A.. |





| RRI | Compound | Leaf (%) | Flower (%) | Immature Fruit (%) | Mature Fruit (%) | Seed (%) | IM |
|---|---|---|---|---|---|---|---|
| 1032 | α-Pinene | 6.3 | 8.0 | 4.8 | 3.8 | 1.0 | RRI, MS |
| 1063 | Ethyl 2-methylbutyrate | - | - | - | - | 1.5 | MS |
| 1076 | Camphene | 0.1 | 0.7 | 1.1 | 1.6 | - | RRI, MS |
| 1100 | Isobutyl isobutyrate | - | - | - | - | 0.7 | MS |
| 1118 | β-Pinene | 23.0 | 32.3 | 12.7 | 6.9 | 1.2 | RRI, MS |
| 1132 | Sabinene | - | 0.3 | - | - | - | RRI, MS |
| 1151 | Propyl 2-methylbutyrate | - | - | - | - | 1.0 | MS |
| 1174 | Myrcene | - | 0.4 | - | - | 0.6 | RRI, MS |
| 1176 | α-Phellandrene | - | - | - | - | 1.1 | RRI, MS |
| 1185 | Isobutyl 2-methylbutyrate | - | - | - | - | 2.4 | MS |
| 1198 | Isobutyl 3-methylbutyrate | - | - | - | - | 1.9 | MS |
| 1203 | Limonene | 1.1 | 1.0 | 1.7 | 1.4 | 1.0 | RRI, MS |
| 1213 | 1,8-Cineole | 4.1 | 4.4 | 4.5 | 12.2 | - | RRI, MS |
| 1218 | β-Phellandrene | - | - | - | - | 7.3 | RRI, MS |
| 1241 | Butyl-2-methylbutyrate | - | - | - | - | 1.3 | MS |
| 1246 | (Z)-β-Ocimene | - | 0.8 | - | - | - | MS |
| 1280 | p-Cymene | 0.4 | 0.3 | 0.8 | 1.3 | 5.5 | RRI, MS |
| 1290 | Terpinolene | - | - | 0.5 | 0.2 | - | RRI, MS |
| 1286 | 2-Methyl butyl 2-methylbutyrate | - | - | - | - | 1.3 | MS |
| 1299 | 2-Methylbutyl isovalerate | - | - | - | - | 0.8 | MS |
| 1429 | Perillene | - | - | - | - | 0.4 | MS |
| 1450 | trans-Linalool oxide (Furanoid) | - | 0.8 | - | - | - | MS |
| 1452 | α,p-Dimethylstyrene | 0.2 | - | - | 0.3 | - | MS |
| 1463 | 1-Heptanol | - | - | - | - | 0.5 | MS |
| 1493 | α-Ylangene | - | - | - | - | 0.7 | MS |
| 1497 | α-Copaene | - | - | 0.2 | 0.2 | 1.6 | RRI, MS |
| 1532 | Camphor | - | - | - | 0.3 | - | RRI, MS |
| 1553 | Linalool | 0.7 | 4.7 | 0.5 | 0.8 | - | RRI, MS |
| 1562 | 1-Octanol | - | - | - | - | 6.2 | MS |
| 1586 | Pinocarvone | 0.3 | - | 0.3 | 1.8 | - | RRI, MS |
| 1591 | Bornyl acetate | - | 0.2 | 2.8 | 4.1 | 0.4 | RRI, MS |
| 1594 | trans-β-Bergamotene | 0.3 | 1.2 | 0.7 | 0.4 | 1.7 | MS |
| 1600 | β-Elemene | 13.6 | 7.7 | 12.9 | 5.7 | - | MS |
| 1611 | Terpinen-4-ol | 0.8 | 0.6 | 1.1 | 0.8 | - | RRI, MS |
| 1612 | β-Caryophyllene | 3.4 | 1.1 | 7.9 | 2.9 | 8.8 | RRI, MS |
| 1648 | Myrtenal | 1.1 | 0.9 | 0.7 | 4.0 | - | MS |
| 1661 | trans-Pinocarvyl acetate | 3.8 | 3.3 | 1.5 | 2.3 | - | MS |
| 1669 | Sesquisabinene | - | - | - | - | 1.7 | MS |
| 1670 | trans-Pinocarveol | 0.9 | 0.8 | 0.5 | 2.4 | - | RRI, MS |
| 1687 | α-Humulene | 0.8 | 0.4 | 1.4 | 0.6 | 1.0 | RRI, MS |
| 1687 | Methyl chavicol | - | - | - | - | 2.6 | RRI, MS |
| 1688 | Selina-4,11-diene | 0.4 | 0.3 | 1.1 | - | - | MS |
| 1695 | (E)-β-Farnesene | - | - | - | - | 0.4 | MS |
| 1704 | Myrtenyl acetate | - | - | - | - | - | MS |
| 1704 | γ-Muurolene | - | - | 0.6 | 0.5 | 2.7 | MS |
| 1706 | α-Terpineol | 2.4 | 2.5 | 5.1 | 3.9 | - | RRI, MS |
| 1719 | Borneol | 0.2 | - | 0.7 | 1.2 | - | RRI, MS |
| 1725 | Verbenone | - | - | - | 0.7 | - | RRI, MS |
| 1726 | Germacrene D | - | 0.3 | - | - | - | RRI, MS |
| 1740 | α-Muurolene | - | - | - | - | 1.6 | MS |
| 1742 | Geranial | - | 0.5 | - | - | - | RRI, MS |
| 1742 | β-Selinene | 1.5 | 1.2 | 2.9 | 1.6 | 0.9 | MS |
| 1744 | α-Selinene | 1.4 | 0.9 | 2.3 | 1.5 | 0.7 | MS |
| 1766 | 1-Decanol | - | - | - | - | 3.3 | MS |
| 1773 | δ-Cadinene | - | 0.3 | 1.3 | 0.3 | 4.0 | MS |
| 1776 | γ-Cadinene | - | 0.1 | - | 0.5 | 2.0 | MS |
| 1784 | (E)-α-Bisabolene | 0.4 | 0.6 | 1.2 | 0.4 | 0.8 | MS |
| 1799 | Cadina-1,4-diene | - | - | - | - | 0.3 | MS |
| 1804 | Myrtenol | 1.5 | 1.4 | 0.5 | 2.2 | - | MS |
| 1808 | Nerol | - | 0.1 | - | - | - | RRI, MS |
| 1849 | Calamenene | - | - | 0.5 | 0.4 | 1.8 | MS |
| 1857 | Geraniol | - | 2.5 | - | - | - | RRI, MS |
| 1864 | p-Cymen-8-ol | 1.0 | 0.4 | - | 0.6 | - | RRI, MS |
| 1872 | cis-Myrtanol | - | tr | - | - | - | MS |
| 1879 | trans-Myrtanol | - | 0.2 | - | - | - | MS |
| 1941 | α-Calacorene | - | - | 0.2 | 0.2 | 0.9 | MS |
| 1948 | trans-Jasmone | - | 1.0 | - | - | - | MS |
| 2008 | Caryophyllene oxide | 3.9 | 0.9 | 1.8 | 7.2 | 1.9 | RRI, MS |
| 2029 | Perilla alcohol | - | - | - | 0.3 | - | MS |
| 2050 | (E)-Nerolidol | 1.3 | 1.7 | 0.6 | 0.4 | 1.2 | RRI, MS |
| 2071 | Humulene epoxide-II | 0.6 | 0.2 | 0.3 | 1.2 | - | MS |
| 2080 | Junenol (=Eudesm-4(15)-en-6-ol) | - | 0.2 | - | - | - | MS |
| 2100 | Heneicosane | - | 0.5 | - | - | - | RRI, MS |
| 2186 | Eugenol | - | - | - | - | 1.3 | RRI, MS |
| 2187 | T-Cadinol | 0.3 | 0.5 | 0.7 | 0.2 | 0.3 | MS |
| 2209 | T-Muurolol | 0.3 | 0.8 | 0.9 | 0.7 | 0.1 | MS |
| 2226 | Methyl hexadecanoate | 0.4 | - | 0.6 | - | 0.3 | RRI, MS |
| 2219 | δ-Cadinol | - | - | 0.2 | - | - | MS |
| 2255 | α-Cadinol | 0.3 | 1.3 | 1.4 | 0.7 | 0.3 | MS |
| 2256 | Cadalene | - | - | - | - | 0.7 | MS |
| 2262 | Ethyl hexadecanoate | - | - | - | - | 0.7 | MS |
| 2269 | Guaia-6,10(14)-dien-4β-ol | 0.3 | - | 0.6 | 0.7 | - | MS |
| 2273 | Selin-11-en-4α-ol | 1.0 | 1.8 | 4.0 | 1.5 | - | MS |
| 2300 | Tricosane | - | 0.6 | - | - | - | RRI, MS |
| 2316 | Caryophylla-2(12),6(13)-dien-5β-ol (=Caryophylladienol I) | - | - | - | 1.4 | - | MS |
| 2353 | Chavicol | - | - | - | - | 0.7 | MS |
| 2369 | (2E,6E)-Farnesol | - | 2.3 | - | - | - | MS |
| 2389 | Caryophylla-2(12),6-dien-5β-ol (=Caryophyllenol I) | - | - | - | 1.8 | - | MS |
| 2456 | (Z)-9-Methyl octadecanoate (=Methyl oleate) | - | - | 1.7 | - | 0.5 | RRI, MS |
| 2509 | (Z.Z)-9,12-methyl octadecadienoate (=Methyl linoleate) | - | - | - | - | 0.7 | RRI, MS |
| 2931 | Hexadecanoic acid | - | - | 0.5 | - | 2.9 | RRI, MS |
| Monoterpene hydrocarbons | 30.9 | 43.8 | 21.6 | 15.2 | 17.7 | ||
| Oxygenated monoterpenes | 16.8 | 23.3 | 18.2 | 36.9 | 0.4 | ||
| Sesquiterpene hydrocarbons | 21.4 | 13.5 | 32.0 | 15.5 | 31.5 | ||
| Oxygenated Sesquiterpenes | 8.4 | 10.3 | 11.7 | 16.2 | 4.6 | ||
| Fatty acids and their esters | 0.4 | - | 2.8 | - | 5.1 | ||
| Aliphatic esters | - | - | - | - | 10.9 | ||
| others | 0.2 | 2.1 | - | 0.3 | 15.0 | ||
| Total | 78.1 | 93.0 | 86.3 | 84.1 | 85.2 |
| Essential oil | LC50 (95%CI) * | LC90 (95%CI) | χ2 | DF |
|---|---|---|---|---|
| Immature fruit | 49.4 (39.4–64.2) | 135.9 (96.5–244.2) | 43.0 | 48 |
| Mature fruit | 48.9 (42.3–56.9) | 116.9 (94.6–158.3) | 83.3 | 48 |
| 1-Decanol | 4.8 (4.2–5.5) | 10.2 (8.5–13.2) | 83.2 | 48 |
| 1-Octanol | 34.3 (30.3–38.7) | 63.9 (54.4–80.5) | 73.7 | 48 |
| Leaf | 20% ** | |||
| Flower | 0% | |||
| Seed | 50% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Tabanca, N.; Demirci, B.; Raman, V.; Budel, J.M.; Baser, K.H.C.; Khan, I.A. Insecticidal and Biting Deterrent Activities of Magnolia grandiflora Essential Oils and Selected Pure Compounds against Aedes aegypti. Molecules 2020, 25, 1359. https://doi.org/10.3390/molecules25061359
Ali A, Tabanca N, Demirci B, Raman V, Budel JM, Baser KHC, Khan IA. Insecticidal and Biting Deterrent Activities of Magnolia grandiflora Essential Oils and Selected Pure Compounds against Aedes aegypti. Molecules. 2020; 25(6):1359. https://doi.org/10.3390/molecules25061359
Chicago/Turabian StyleAli, Abbas, Nurhayat Tabanca, Betul Demirci, Vijayasankar Raman, Jane M. Budel, K. Hüsnü Can Baser, and Ikhlas A. Khan. 2020. "Insecticidal and Biting Deterrent Activities of Magnolia grandiflora Essential Oils and Selected Pure Compounds against Aedes aegypti" Molecules 25, no. 6: 1359. https://doi.org/10.3390/molecules25061359
APA StyleAli, A., Tabanca, N., Demirci, B., Raman, V., Budel, J. M., Baser, K. H. C., & Khan, I. A. (2020). Insecticidal and Biting Deterrent Activities of Magnolia grandiflora Essential Oils and Selected Pure Compounds against Aedes aegypti. Molecules, 25(6), 1359. https://doi.org/10.3390/molecules25061359