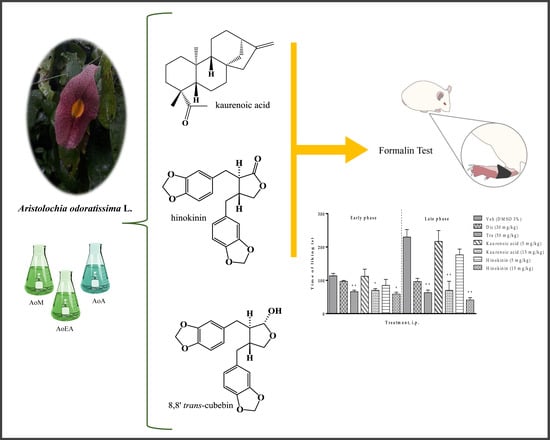
Antinociceptive Effect of Hinokinin and Kaurenoic Acid Isolated from Aristolochia odoratissima L.
Abstract
:1. Introduction
2. Results
2.1. Antinociceptive Effect of the Extracts of Aristolochia odoratissima
2.2. Antinociceptive of Kaurenoic Acid (1) and Hinokinin (2) Isolated from Aristolochia odoratissima
2.3. Characterization by NMR of the Compounds (1–3)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Isolation and Identification of the Compounds
4.4. Experimentation Animals
4.5. Drugs and Reagents
4.6. The Formalin Test
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Almeida, R.N.; Navarro, D.S.; Barbosa-Filho, J.M. Plants with central analgesic activity. Phytomedicine 2001, 8, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Goucke, C.R.; Chaudakshetrin, P. Pain: A Neglected Problem in the Low-Resource Setting. Anesth. Analg. 2018, 126, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health. 2011, 11, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel, H.L.M.; Steagall, P.V. Adjuvant Analgesics in Acute Pain Management. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in the Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Emery, M.A.; Eitan, S. Members of the same pharmacological family are not alike: Different opioids, different consequences, hope for the opioid crisis? Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 428–449. [Google Scholar] [CrossRef]
- Ghelardini, C.; Di Cesare Mannelli, L.; Bianchi, E. The pharmacological basis of opioids. Clin. Cases Miner. Bone Metab. 2015, 12, 219–221. [Google Scholar] [CrossRef]
- Kenny, G.N. Potential renal, haematological and allergic adverse effects associated with nonsteroidal anti-inflammatory drugs. Drugs 1992, 44, 31–36. [Google Scholar] [CrossRef]
- Carter, G.T.; Duong, V.; Ho, S.; Ngo, K.C.; Greer, C.L.; Weeks, D.L. Side effects of commonly prescribed analgesic medications. Phys. Med. Rehabil. Clin. N Am. 2014, 25, 457–470. [Google Scholar] [CrossRef]
- Candiotti, K.A.; Gitlin, M.C. Review of the effect of opioid-related side effects on the undertreatment of moderate to severe chronic non-cancer pain: Tapentadol, a step toward a solution? Curr. Med. Res. Opin. 2010, 26, 1677–1684. [Google Scholar] [CrossRef]
- Batista, F.L.A.; Lima, L.M.G.; Abrante, I.A.; de Araujo, J.I.F.; Batista, F.L.A.; Abrante, I.A.; Magalhãesa, E.A.; de Lima, D.R.; Maria da Conceição, L.L.; do Prado, B.S.; et al. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed Pharmacother. 2018, 108, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.S.; Heimfarth, L.; Santos, K.A.; Guimarães, A.G.; Picot, L.; Almeida, J.R.G.S.; Quintans, J.S.S.; Quintans-Júnior, L.J. Terpenes as possible drugs for the mitigation of arthritic symptoms—A systematic review. Phytomedicine 2019, 57, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Coy-Barrera, E.D.; Cuca-Suarez, L.E. In vitro anti-inflammatory effects of naturally-occurring compounds from two Lauraceae plants. Acad Bras Ciênc. 2011, 83, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Gómez Álvarez, R. Plantas medicinales en una aldea del estado de Tabasco, México. Rev. Fitotec. Mex. 2012, 35, 43–49. [Google Scholar]
- Usubillaga, A.; Khouri, N.; Rojas, L.B. Essential Oil from the Leaves of Aristolochia odoratissima L. J. Essent. Oil Res. 2001, 13, 128–129. [Google Scholar] [CrossRef]
- Usubillaga, A.; Khouri, N.; Cedillo-Vaz, S.; Yibirin, E. Anti-snake venom effect of Aristolochia odoratissima L. aqueous extract on mice. Acta Hortic. 2005, 677, 88–89. [Google Scholar] [CrossRef] [Green Version]
- Usubillaga, A.; Khouri, N.; Bruno-Colmenárez, J.; Díaz de Delgado, G. Cubebin, a lignan isolated. from Aristolochia odoratissima L. Acta Crystallogr. Sect. E 2007, 63, o2046–o2047. [Google Scholar] [CrossRef]
- Lopes, L.M.X.; Bolzani, V.D.S.; Trevisan, L.M.V.; Grigolli, T.M. Terpenes from Aristolochia triangularis. Phytochemistry 1990, 29, 660–662. [Google Scholar] [CrossRef]
- Wenkert, E.; Gottlieb, H.E.; Gottlieb, O.R.; Pereira, M.O.D.S.; Formiga, M.D. 13C NMR spectroscopy of neolignans. Phytochemistry 1976, 15, 1547–1551. [Google Scholar] [CrossRef]
- De Pascoli, I.C.; Nascimento, I.R.; Lopes, L.M.X. Configurational analysis of cubebins and bicubebin from Aristolochia lagesiana and Aristolochia pubescens. Phytochemistry 2006, 67, 735–742. [Google Scholar] [CrossRef]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified formalin test: Characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Desai, D.C.; Jacob, J.; Almeida, A.; Kshirsagar, R.; Manju, S.L. Isolation, structural elucidation and anti-inflammatory activity of astragalin, (-)hinokinin, aristolactam I and aristolochic acids (I & II) from Aristolochia indica. Nat. Prod. Res. 2014, 28, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Sartorelli, P.; Carvalho, C.S.; Reimão, J.Q.; Lorenzi, H.; Tempone, A.G. Antitrypanosomal activity of a diterpene and lignans isolated from Aristolochia cymbifera. Planta Med. 2010, 76, 1454–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Arellanes, A.; León-Díaz, R.; Meckes, M.; Tapia, A.; Molina-Salinas, G.M.; Luna-Herrera, J.; Yépez-Mulia, L. Antiprotozoal and Antimycobacterial Activities of Pure Compounds from Aristolochia elegans Rhizomes. Evid Based Complement. Alternat. Med. 2012, 2012, 593403. [Google Scholar] [CrossRef] [Green Version]
- Messiano, G.B.; Vieira, L.; Machado, M.B.; Lopes, L.M.; de Bortoli, S.A.; Zukerman-Schpector, J. Evaluation of insecticidal activity of diterpenes and lignans from Aristolochia malmeana against Anticarsia gemmatalis. J. Agric. Food Chem. 2008, 56, 2655–2659. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Tengku Mohamad, T.A.; Shaik Mossadeq, W.M.; Moin, S.; Yusof, M.; Mokhtar, A.F.; Zakaria, Z.A.; Israf, D.A.; Lajis, N. Antinociceptive activity of the essential oil of Zingiber zerumbet. Planta Med. 2010, 76, 107–112. [Google Scholar] [CrossRef]
- Dalenogare, D.P.; Ferro, P.R.; De Pra, S.D.T.; Rigo, F.K.; de David Antoniazzi, C.T.; de Almeida, A.S.; Damiani, A.P.; Strapazzon, G.; de Oliveira Sardinha, T.T.; Galvani, N.C.; et al. Antinociceptive activity of Copaifera officinalis Jacq. L oil and kaurenoic acid in mice. Inflammopharmacology 2019, 27, 829–844. [Google Scholar] [CrossRef]
- Block, L.C.; Santos, A.R.S.; de Souza, M.M.; Scheidt, C.; Yunes, R.A.; Santos, M.A.; Monache, F.D.; Filho, V.C. Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J. Ethnopharmacol. 1998, 61, 85–89. [Google Scholar] [CrossRef]
- Mizokami, S.S.; Arakawa, N.S.; Ambrosio, S.R.; Zarpelon, A.C.; Casagrande, R.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Kaurenoic acid from Sphagneticola trilobata Inhibits Inflammatory Pain: Effect on cytokine production and activation of the NO-cyclic GMP-protein kinase G-ATP-sensitive potassium channel signaling pathway. J. Nat. Prod. 2012, 75, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.A.; Gurgel, L.A.; Silva, R.M.; Tomé, A.R.; Gramosa, N.V.; Silveira, E.R.; Santos, F.A.; Rao, V.S.N. Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffi on acetic acid-induced colitis in rats. Vascul. Pharmacol. 2002, 39, 303–307. [Google Scholar] [CrossRef]
- Cavalcanti, B.; Costa-Lotufo, L.; Moraes, M.O.; Burbano, R.R.; Silveira, E.R.; Cunha, K.M.; Rao, V.S.N.; Moura, D.J.; Rosa, R.M.; Henriques, J.A.P.; et al. Genotoxicity evaluation of kaurenoic acid, a bioactive diterpenoid present in Copaiba oil. Food Chem. Toxicol. 2006, 44, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, M.C.; Pelosi, A.; Curini, M. Hinokinin, an emerging bioactive lignan. Molecules 2014, 19, 14862–14878. [Google Scholar] [CrossRef]
- Lima, L.M.; Perazzo, F.F.; Tavares Carvalho, J.C.; Bastos, J.K. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J. Pharm. Pharmacol. 2007, 59, 1151–1158. [Google Scholar] [CrossRef]
- Borges, A.; Casoti, R.; e Silva, M.L.A.; da Cunha, N.L.; da Rocha Pissurno, A.P.; Kawano, D.F.; da Silva de Laurentiz, R. COX Inhibition Profiles and Molecular Docking Studies of the Lignan Hinokinin and Some Synthetic Derivatives. Mol. Inform. 2018, 37, e1800037. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Boakye-Gyasi, E.; Kasanga, E.A.; Ameyaw, E.O.; Abotsi, W.K.M.; Biney, R.P.; Agyare, C.; Woode, E. An isobolographic analysis of the anti-nociceptive effect of geraniin in combination with morphine or diclofenac. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 201–209. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Huang, Y.; Qu, Y.; Zhang, G.; Wang, D. Analgesic effects of Marasmius androsaceus mycelia ethanol extract and possible mechanisms in mice. Braz. J. Med. Biol. Res. 2018, 51, e7124. [Google Scholar] [CrossRef]
- Romero, A.; Miranda, H.F.; Puig, M.M. Analysis of the opioid–opioid combinations according to the nociceptive stimulus in mice. Pharmacol. Res. 2010, 61, 511–518. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |







| Position | δ 13C 2 | δ 1H (J in Hz) 2 | δ 13C 3 | δ 1H (J in Hz) 3 |
|---|---|---|---|---|
| 1 | 131.5 | 134.6 | ||
| 2 | 109.3 | 6.63 (1H, d, 1.4) | 109.5 | 6.58 (1H, s, br) |
| 3 | 147.8 | 147.9 | ||
| 4 | 146.4 | 146.1 | ||
| 5 | 108.2 | 6.73 (1H, d, 7.7) | 108.3 | 6.69 (1H, d, 7.7) |
| 6 | 122.1 | 6.60 (1H, dd, 1.4, 7.7) | 122.1 | 6.55 (1H, d, br, 7.7) |
| 7 | 38.3 | a 3.0 (1H, dd, 5.1, 14.3) b 2.84 (1H, dd, 7.3, 14.3) | 39.3 | a 2.53 (1H, dd, 6.2, 13.9) b 2.53 (1H, dd, 7.3, 13.5) |
| 8 | 46.4 | 2.53 (1H, ddd, 5.1,7.7, 8.0) | 52.6 | 2.12 (1H, m) |
| 9 | 178.3 | 104.6 | 5.14 (1H, d, 1.6) | |
| 1′ | 131.2 | 133.8 | ||
| 2′ | 108.7 | 6.47 (1H, d, 1.8) | 109.2 | 6.51 (1H, s, br) |
| 3′ | 147.8 | 147.9 | ||
| 4′ | 146.2 | 146.1 | ||
| 5′ | 108.1 | 6.7 (1H, d, 8.4) | 108.4 | 6.69 (1H, d, 7.7) |
| 6′ | 121.4 | 6.47 (1H, dd, 2.2, 8) | 121.7 | 6.50 (1H, d, br, 8.0) |
| 7′ | 34.7 | a 2.60 (1H, dd, 9.9, 17.2) b 2.47 (1H, dd, 8.4, 16.8) | 38.7 | a 2.64 (1H, dd, 7.7, 13.9) b 2.43 (1H, dd, 7.3, 13.5) |
| 8′ | 41.2 | 2.46 (1H, m) | 46.0 | 2.12 (1H, m) |
| 9′ | 71.0 | a 4.13 (1H, dd, 6.9, 9.5) b 3.86 (1H, dd, 7.3, 9.1) | 72.3 | a 3.92 (1H, dd, 7.3, 8.4) b 3.41 (1H, dd, 8.0, 8.4) |
| 1-O-CH2-O | 100.9 | 5.93 (2H, m) | 101.1 | 5.93 (2H, m) |
| 2-O-CH2-O | 100.9 | 5.93 (2H, m) | 101.1 | 5.90 (2H, m) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel-Ruiz, R.M.; Córdova-de la Cruz, M.; González-Cortázar, M.; Zamilpa, A.; Gómez-Rivera, A.; López-Rodríguez, R.; Lobato-García, C.E.; Blé-González, E.A. Antinociceptive Effect of Hinokinin and Kaurenoic Acid Isolated from Aristolochia odoratissima L. Molecules 2020, 25, 1454. https://doi.org/10.3390/molecules25061454
Montiel-Ruiz RM, Córdova-de la Cruz M, González-Cortázar M, Zamilpa A, Gómez-Rivera A, López-Rodríguez R, Lobato-García CE, Blé-González EA. Antinociceptive Effect of Hinokinin and Kaurenoic Acid Isolated from Aristolochia odoratissima L. Molecules. 2020; 25(6):1454. https://doi.org/10.3390/molecules25061454
Chicago/Turabian StyleMontiel-Ruiz, Rosa Mariana, Marcos Córdova-de la Cruz, Manasés González-Cortázar, Alejandro Zamilpa, Abraham Gómez-Rivera, Ricardo López-Rodríguez, Carlos Ernesto Lobato-García, and Ever A. Blé-González. 2020. "Antinociceptive Effect of Hinokinin and Kaurenoic Acid Isolated from Aristolochia odoratissima L." Molecules 25, no. 6: 1454. https://doi.org/10.3390/molecules25061454
APA StyleMontiel-Ruiz, R. M., Córdova-de la Cruz, M., González-Cortázar, M., Zamilpa, A., Gómez-Rivera, A., López-Rodríguez, R., Lobato-García, C. E., & Blé-González, E. A. (2020). Antinociceptive Effect of Hinokinin and Kaurenoic Acid Isolated from Aristolochia odoratissima L. Molecules, 25(6), 1454. https://doi.org/10.3390/molecules25061454