Ionic Liquids and Ohmic Heating in Combination for Pd-Catalyzed Cross-Coupling Reactions: Sustainable Synthesis of Flavonoids
Abstract
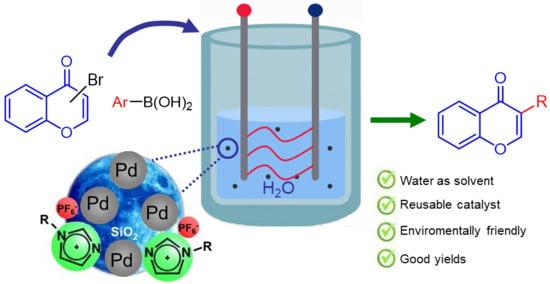
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Representative Procedure for Catalyst Preparation
3.2. General Procedure for the Suzuki–Miyaura Reaction.
4. Conclusions
Supplementary Files
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
References
- Seechurn, C.C.C.J.M.; Kitching, O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Sasaki, T.; Zhong, C.; Tada, M.; Iwasawa, Y. Immobilized metal ion-containing ionic liquids: Preparation, structure and catalytic performance in Kharasch addition reaction. Chem. Commun. 2005, 2506–2608. [Google Scholar] [CrossRef]
- Riisager, A.; Fehrmann, R. Imidazolium-based ionic liquids grafted on solid surfaces. Chem. Soc. Rev. 2014, 43, 7171–7187. [Google Scholar] [CrossRef]
- Migowski, P.; Dupont, J. Catalytic applications of metal nanoparticles in imidazolium ionic liquids. Chem.: Eur. J. 2007, 13, 32–39. [Google Scholar] [CrossRef]
- Razzaq, T.; Kappe, C.O. On the energy efficiency of microwave-assisted organic reactions. Chem. Sus. Chem. 2008, 1, 123–132. [Google Scholar] [CrossRef]
- Nuchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis–a critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Pinto, J.; Silva, V.L.M.; Silva, A.M.G.; Silva, A.M.S.; Costa, J.C.S.; Santos, L.M.N.B.F.; Enes, R.; Cavaleiro, J.A.S.; Vicente, A.A.M.O.S.; Teixeira, J.A.C. Ohmic heating as a new efficient process for organic synthesis in water. Green Chem. 2013, 15, 970–975. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Santos, L.M.N.B.F.; Silva, A.M.S. Ohmic heating: An emerging concept in organic synthesis. Chem. Eur. J. 2017, 23, 7853–7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soengas, R.G.; Silva, V.L.M.; Pinto, J.; Rodríguez-Solla, H.; Silva, A.M.S. Ohmic heating and ionic liquids in combination for the indium-promoted synthesis of 1-halo alkenyl compounds: applications to Pd-catalysed cross-coupling reactions. Eur. J. Org. Chem. 2016, 99–107. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S.; Santos, L.M.N.B.F.; Silva, A.M.G.; Pinto, J.; Enes, R.; Cavaleiro, J.A.S.; Vicente, A.A.M.O.S.; Teixeira, J.A.C.; Morais, A.; et al. Reator para síntese química com aquecimento óhmico, método e suas aplicações, PT105908, 27-09-2011.
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612–108622. [Google Scholar] [CrossRef]
- Mirossay, L.; Varinská, L.; Mojžiš, J. Antiangiogenic effect of flavonoids and chalcones: an update. Int. J. Mol. Sci. 2018, 19, 27. [Google Scholar] [CrossRef] [Green Version]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tomé, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araújo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure-activity relationship. J. Enz. Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enz. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, A.M.; Man, T.; Idrissi, I.; Souza, C.C.; Mead, E.; Dunbar, C.; Wolak, J.; Oliveira, M.C.; Evans, D.; Grayson, J.; et al. Discovery of N-methylpiperazinyl flavones as a novel class of compounds with therapeutic potential against Alzheimer’s disease: Synthesis, binding affinity towards amyloid β oligomers (Aβo) and ability to disrupt Aβo-PrPC interactions. Pure Appl. Chem. 2019, 91, 1107–1136. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Laufer, S.; Lima, J.L.F.C.; Fernandes, E. Flavonoids inhibit COX-1 and COX-2 enzymes and cytokine/chemokine production in human whole blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Freitas, M.; Ribeiro, D.; Tomé, S.M.; Silva, A.M.S.; Fernandes, E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur. J. Med. Chem. 2014, 86, 153–164. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Porto, G.; Fernandes, E. Modulation of human neutrophils’ oxidative burst by flavonoids. Eur. J. Med. Chem. 2013, 67, 280–292. [Google Scholar] [CrossRef]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.P.R.; Silva, A.M.S.; Seixas, R.S.G.R.; Pinto, D.C.G.A.; Tomé, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef]
- Silva, C.F.M.; Pinto, D.C.G.A.; Silva, A.M.S. Chromones: A promising ring system for new anti-inflammatory drugs. Chem. Med. Chem. 2016, 11, 2252–2260. [Google Scholar] [CrossRef]
- Springsteel, M.F.; Galietta, L.J.V.; Ma, T.; By, K.; Berger, G.O.; Yang, H.; Dicus, C.W.; Choung, W.; Quan, C.; Shelat, A.A. Benzoflavone activators of the cystic fibrosis transmembrane conductance regulator: Towards a pharmacophore model for the nucleotide-binding domain. Bioorg. Med. Chem. 2003, 11, 4113–4120. [Google Scholar] [CrossRef]
- Ko, S.K.; Jang, H.J.; Kim, E.; Park, S.B. Concise and diversity-oriented synthesis of novel scaffolds embedded with privileged benzopyran motif. Chem. Commun. 2006, 2962–2964. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Kim, S.B.; Sin, K.S.; Kim, S.; Kim, H.P.; Park, H. Synthesis and PGE2 inhibitory activity of vinylated and allylated chrysin analogues. Arch. Pharmacol Res. 2003, 26, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Parasuraman, K.; Subramanian, V.; Dakarapu, R.; Yeleswarapu, K.R. Palladium mediated stereospecific synthesis of 3-enynyl substituted thioflavones/flavones. Tetrahedron Lett. 2004, 45, 2305–2309. [Google Scholar] [CrossRef]
- Pal, M.; Dakarapu, R.; Parasuraman, K.; Subramanian, V.; Yeleswarapu, K.R. Synthesis of isocoumarins via Pd/C-mediated reactions of o-iodobenzoic acid with terminal alkynes. J. Org. Chem. 2005, 70, 7179–7187. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Kim, J.K.; Kang, S.H.; Noh, M.S.; Ha, J.Y.; Choi, J.K.; Lim, K.M.; Lee, C.H.; Chung, S. 2,3-Diarylbenzopyran derivatives as a novel class of selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 413–417. [Google Scholar] [CrossRef]
- Fitzmaurice, R.J.; Etheridge, Z.C.; Jumel, E.; Woolfson, D.N.; Caddick, S. Microwave enhanced palladium catalysed coupling reactions: A diversity-oriented synthesis approach to functionalized flavones. Chem. Commun. 2006, 4814–4816. [Google Scholar] [CrossRef]
- Selepe, M.A.; Van Heerden, F.R. Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules 2013, 18, 4739–4765. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.; Silva, V.L.M.; Silva, A.M.G.; Santos, L.M.N.B.F.; Silva, A.M.S. Ohmic heating-assisted Synthesis of 3-Arylquinolin-4(1H) ones by a Reusable and ligand-free Suzuki-Miyaura reaction in water. J. Org. Chem. 2015, 80, 6649–6659. [Google Scholar] [CrossRef]
- Pinto, J.; Silva, V.L.M.; Santos, L.M.N.B.F.; Silva, A.M.S. Synthesis of (E)-3-Styrylquinolin-4(1H)-ones in water by ohmic heating: A comparison with other methodologies. Eur. J. Org. Chem. 2016, 2888–2896. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sugawara, Y.; Isobe, K.; Hoshi, T.; Suzuki, T. Immobilization of Pd(OAc)2 in ionic liquid on silica: Application to sustainable Mizoroki-Heck reaction. Org. Lett. 2004, 6, 2325–2328. [Google Scholar] [CrossRef]
- Soengas, R.; Navarro, Y.; Iglesias, M.J.; López-Ortiz, F. Immobilized gold nanoparticles prepared from Gold(III)-containing ionic liquids on silica: application to the sustainable synthesis of propargylamines. Molecules 2018, 23, 2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiden, F.; Schünemann, J. Reaction of Formylchromones with Dichloroketene. Arch. Pharm. 1984, 317, 970–972. [Google Scholar] [CrossRef]
- Felpin, F.-X. Practical and efficient Suzuki−Miyaura cross-coupling of 2-iodocycloenones with arylboronic acids catalyzed by recyclable Pd(0)/C. J. Org. Chem. 2005, 70, 8575–8578. [Google Scholar] [CrossRef] [PubMed]
- Vasselin, D.A.; Westwell, A.D.; Matthews, C.S.; Bradshaw, T.D.; Stevens, M.F.G. Structural studies on bioactive compounds. 40.1 Synthesis and biological properties of fluoro-, methoxyl-, and amino-substituted 3-phenyl-4H-1-benzopyran-4-ones and a comparison of their antitumor activities with the activities of related 2-phenylbenzothiazoles. J. Med. Chem. 2006, 49, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Armelin, E.A.; Donate, P.M.; Galembeck, S.E. Effect of the environment on the reactivity of 4’-substituted flavones and isoflavones. Tetrahedron 2000, 56, 5105–5111. [Google Scholar] [CrossRef]
- Davies, S.G.; Mobbs, B.E.; Goodwin, C.J. Substituted 4H-1-benzopyran-4-ones (chromones): Synthesis via palladium-catalysed coupling of their halogeno derivatives with alkenes. J. Chem. Soc. Perkin Trans. 1 1987, 2597–2604. [Google Scholar] [CrossRef]
- Takao, K.; Ishikawa, R.; Sugita, Y. Synthesis and biological evaluation of 3-styrylchromone derivatives as free radical scavengers and α-glucosidase inhibitors. Chem. Pharm. Bull. 2014, 62, 810–815. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds 3a-e are available from the authors. |


| Entry | Solvent | mol% Pd | TBAB (mol%) | T (°C) | Time (h) | Yield (%) a |
|---|---|---|---|---|---|---|
| 1 | bmim[Br] | 1 | - | 100 b | 12 | 34 |
| 2 | bmim[PF6] | 1 | - | 100 b | 12 | 65 |
| 3 | DMF | 1 | - | 100 b | 12 | 69 |
| 4 | DMF/H2O 5:2 | 1 | - | 100 b | 12 | 72 |
| 5 | H2O | 1 | - | 100 b | 12 | 9 |
| 6 | H2O | 1 | 0.1 | 100 b | 12 | 89 |
| 7 | H2O | 1 | 0.1 | 100 c | 1 | 93 |
| 8 | H2O | 0.1 | 0.1 | 100 c | 1 | 92 |
| 9 | H2O | 0.05 | 0.1 | 100 c | 1 | 90 |

| Entry | R1 | R2 | R3 | 1 | R4 | 2 | Product | 3 | Yield (%)a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Br | H | H | 1a | Ph | 2a |  | 3a | 93 |
| 2 | Br | H | 1a | 3,4-OMeC6H3 | 2b |  | 3b | 89 | |
| 3 | Br | H | H | 1a | 4-ClC6H4 | 2c |  | 3c | 91 |
| 4 |  | H | H | 1b | Ph | 2a |  | 3d | 90 |
| 5 |  | H | H | 1b | 3,4-OMeC6H3 | 2b |  | 3e | 85 |
| 6 |  | H | OBn | 1c | 3,4-OMeC6H3 | 2b |  | 3f | 81 |
| 7 |  | H | H | 1d | 3,4-OMeC6H3 | 2b |  | 3g | 82 |
| 8 |  | Cl | H | 1e | 4-ClC6H4 | 2c |  | 3h | 89 |
| 9 |  | H | OBn | 1f | 4-ClC6H4 | 2c |  | 3i | 85 |
| 10 |  | Me | H | 1g | 3,4-OMeC6H3 | 2b |  | 3j | 80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.L.M.; Soengas, R.G.; Silva, A.M.S. Ionic Liquids and Ohmic Heating in Combination for Pd-Catalyzed Cross-Coupling Reactions: Sustainable Synthesis of Flavonoids. Molecules 2020, 25, 1564. https://doi.org/10.3390/molecules25071564
Silva VLM, Soengas RG, Silva AMS. Ionic Liquids and Ohmic Heating in Combination for Pd-Catalyzed Cross-Coupling Reactions: Sustainable Synthesis of Flavonoids. Molecules. 2020; 25(7):1564. https://doi.org/10.3390/molecules25071564
Chicago/Turabian StyleSilva, Vera L. M., Raquel G. Soengas, and Artur M. S. Silva. 2020. "Ionic Liquids and Ohmic Heating in Combination for Pd-Catalyzed Cross-Coupling Reactions: Sustainable Synthesis of Flavonoids" Molecules 25, no. 7: 1564. https://doi.org/10.3390/molecules25071564
APA StyleSilva, V. L. M., Soengas, R. G., & Silva, A. M. S. (2020). Ionic Liquids and Ohmic Heating in Combination for Pd-Catalyzed Cross-Coupling Reactions: Sustainable Synthesis of Flavonoids. Molecules, 25(7), 1564. https://doi.org/10.3390/molecules25071564