Preliminary Study on the Differences in Hydrocarbons Between Phosphine-Susceptible and -Resistant Strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) Using Direct Immersion Solid-Phase Microextraction Coupled with GC-MS
Abstract
:1. Introduction
2. Results
2.1. Resistance Factor
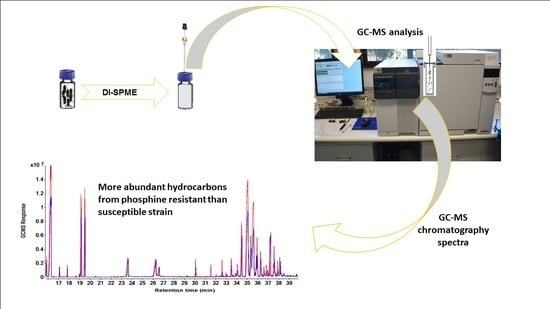
2.2. Hydrocarbons Profiles of Susceptible and Resistant Strains
3. Discussion
3.1. Resistance Factor
3.2. Hydrocarbons of Susceptible and Resistant Strains
4. Materials and Methods
4.1. The Insect Culture
4.2. Generation of Phosphine Gas and Determination of Resistance Factor
4.3. Chemical Reagents and Apparatuses
4.4. GC-MS Instrument and Analytical Conditions
4.5. The Extraction and Analytical Procedures
4.6. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donahaye, E.J.; Bell, C.; Jayes, D.; Noyas, R.; Phillips, T.W. Integrated pest management strategies used in stored grains in Brazil to manage phosphine resistance. In Proceedings of the International Conference Controlled Atmosphere and Fumigation in Stored Product, Gold Coast, Australia, 8–13 August 2007; pp. 293–300. [Google Scholar]
- Edde, P.A. Review of the biology and control of Rhyzopertha dominica (F.) the lesser grain borer. J. Stored Prod. Res. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Collins, P. Resistance to chemical treatments in insect pests of stored grain and its management. In Proceedings of the 9th International Working Conference on Stored Product Protection, São Paulo, Brazil, 15–18 October 2006. [Google Scholar]
- Wang, D.; Collins, P.; Gao, X. Optimising indoor phosphine fumigation of paddy rice bag-stacks under sheeting for control of resistant insects. J. Stored Prod. Res. 2006, 42, 207–217. [Google Scholar] [CrossRef]
- Benhalima, H.; Chaudhry, M.; Mills, K.; Price, N. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 2004, 40, 241–249. [Google Scholar] [CrossRef]
- Chaudhry, M. Phosphine resistance. Pesticide Outlook 2000, 11, 88–91. [Google Scholar] [CrossRef]
- Chaudhry, M.Q.; Price, N.R. Comparison of the oxidant damage induced by phosphine and the uptake and tracheal exchange of 32P-radiolabelled phosphine in the susceptible and resistant strains of Rhyzopertha dominica (F.)(Coleoptera: Bostrychidae). Pestic. Biochem. Phys. 1992, 42, 167–179. [Google Scholar] [CrossRef]
- Pimentel, M.; Faroni, L.; Guedes, R.; Neto, A.; Garcia, F. Phosphine resistance, respiration rate and fitness consequences in Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). In Proceedings of the 9th International Working Conference on Stored Product Protection, São Paulo, Brazil, 15–18 October 2006; pp. 344–351. [Google Scholar]
- Pino Moreno, J.; Ganguly, A. Determination of fatty acid content in some edible insects of Mexico. J. Ins. Food Feed 2016, 2, 37–42. [Google Scholar] [CrossRef]
- Cohen, E.; Moussian, B. Insect Hydrocarbons: Biochemistry and Chemical Ecology. In Extracellular Composite Matrices in Arthropods; Springer: Basel, Switzerland, 2016; pp. 221–252. [Google Scholar]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Stepnowski, P. Cuticular lipids of insects as potential biofungicides: Methods of lipid composition analysis. Anal. Bioanal. Chem. 2011, 399, 3177–3191. [Google Scholar] [CrossRef]
- Lockey, K.H. Hydrocarbons of adult Tribolium castaneum Hbst. and Tribolium confusum Duv.(Coleoptera: Tenebrionidae). Comp. Biochem. Physiol. Part B Comp. Biochem. 1978, 61, 401–407. [Google Scholar] [CrossRef]
- Toolson, E.C.; Kuper-Simbrón, R. Laboratory evolution of epicuticular hydrocarbon composition and cuticular permeability in Drosophila pseudoobscura: Effects on sexual dimorphism and thermal-acclimation ability. Evolution 1989, 43, 468–473. [Google Scholar]
- Gołębiowski, M.; Maliński, E.; Nawrot, J.; Stepnowski, P. Identification and characterization of surface lipid components of the dried-bean beetle Acanthoscelides obtectus (Say)(Coleoptera: Bruchidae). J. Stored Prod. Res. 2008, 44, 386–388. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Maliński, E.; Boguś, M.I.; Kumirska, J.; Stepnowski, P. The cuticular fatty acids of Calliphora vicina, Dendrolimus pini and Galleria mellonella larvae and their role in resistance to fungal infection. Insect Biochem. Mol. Biol. 2008, 38, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Maliński, E.; Nawrot, J.; Szafranek, J.; Stepnowski, P. Identification of the cuticular lipid composition of the Western Flower Thrips Frankliniella occidentalis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Cerkowniak, M.; Puckowski, A.; Stepnowski, P.; Gołębiowski, M. The use of chromatographic techniques for the separation and the identification of insect lipids. J. Chromatogr. B 2013, 937, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Roux, E.; Sreng, L.; Provost, E.; Roux, M.; Clement, J.L. Cuticular hydrocarbon profiles of dominant versus subordinate male Nauphoeta cinerea cockroaches. J. Chem. Ecol. 2002, 28, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Sledge, M.F.; Moneti, G.; Pieraccini, G.; Turillazzi, S. Use of solid-phase microextraction in the investigation of chemical communication in social wasps. J. Chromatogr. A 2000, 873, 73–77. [Google Scholar] [CrossRef]
- Bland, J.M.; Osbrink, W.L.; Cornelius, M.L.; Lax, A.R.; Vigo, C.B. Solid-phase microextraction for the detection of termite cuticular hydrocarbons. J. Chromatogr. A 2001, 932, 119–127. [Google Scholar] [CrossRef]
- Tentschert, J.; Bestmann, H.; Heinze, J. Cuticular compounds of workers and queens in two Leptothorax ant species—a comparison of results obtained by solvent extraction, solid sampling, and SPME. Chemoecology 2002, 12, 15–21. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Moreira, J.A.; Ray, A.M.; Millar, J.G.; Hanks, L.M. (Z)-9-Nonacosene—major component of the contact sex pheromone of the beetle Megacyllene caryae. J. Chem. Ecol. 2006, 32, 435–451. [Google Scholar] [CrossRef]
- De Pasquale, C.; Guarino, S.; Peri, E.; Alonzo, G.; Colazza, S. Investigation of cuticular hydrocarbons from Bagrada hilaris genders by SPME/GC-MS. Anal. Bioanal. Chem. 2007, 389, 1259–1265. [Google Scholar] [CrossRef]
- Alnajim, I.; Du, X.; Lee, B.; Agarwal, M.; Liu, T.; Ren, Y. New method of analysis of lipids in Tribolium castaneum (Herbst) and Rhyzopertha dominica (Fabricius) insects by direct immersion solid-phase microextraction (DI-SPME) coupled with GC–MS. Insects 2019, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Al-Khshemawee, H.; Du, X.; Agarwal, M.; Yang, J.O.; Ren, Y. Application of direct immersion Solid-Phase Microextraction (DI-SPME) for understanding biological changes of Mediterranean fruit fly (Ceratitis capitata) during mating procedures. Molecules 2018, 23, 2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocak, E.; Schlipalius, D.; Kaur, R.; Tuck, A.; Ebert, P.; Collins, P.; Yılmaz, A. Determining phosphine resistance in rust red flour beetle, Tribolium castaneum (Herbst.)(Coleoptera: Tenebrionidae) populations from Turkey. Turk. J. Entomol. 2015, 39, 129–136. [Google Scholar]
- Schlipalius, D.I.; Valmas, N.; Tuck, A.G.; Jagadeesan, R.; Ma, L.; Kaur, R.; Goldinger, A.; Anderson, C.; Kuang, J.; Zuryn, S.A. Core metabolic enzyme mediates resistance to phosphine gas. Science 2012, 338, 807–810. [Google Scholar] [CrossRef] [PubMed]
- FAO. Recommended methods for the detection and measurement of resistance of agricultural pests to pesticides. Tentative method for adults of some major pest species of stored cereals, with methyl bromide and phosphine. FAO Method No. 16. Plant Prot. Bull. 1975, 23, 12–25. [Google Scholar]
- Jagadeesan, R.; Collins, P.J.; Daglish, G.J.; Ebert, P.R.; Schlipalius, D.I. Phosphine resistance in the rust red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae): Inheritance, gene interactions and fitness costs. PLoS ONE 2012, 7, e31582. [Google Scholar] [CrossRef] [Green Version]
- Mau, Y.S.; Collins, P.J.; Daglish, G.J.; Nayak, M.K.; Ebert, P.R. The rph2 gene is responsible for high level resistance to phosphine in independent field strains of Rhyzopertha dominica. PLoS ONE 2012, 7, e34027. [Google Scholar] [CrossRef]
- Nakakita, H.; Kuroda, J. Differences in phosphine uptake between susceptible and resistant strains of insects. J. Pestic. Sci. 1986, 11, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Zuryn, S.; Kuang, J.; Ebert, P. Mitochondrial modulation of phosphine toxicity and resistance in Caenorhabditis elegans. Toxicol. Sci. 2008, 10, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Price, N. The effect of phosphine on respiration and mitochondrial oxidation in susceptible and resistant strains of Rhyzopertha dominica. Insect Biochem. 1980, 10, 65–71. [Google Scholar] [CrossRef]
- Pratt, S.J. A new measure of uptake: Desorption of unreacted phosphine from susceptible and resistant strains of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2003, 39, 507–520. [Google Scholar] [CrossRef]
- Price, N.R.; Dance, S.J. Some biochemical aspects of phosphine action and resistance in three species of stored product beetles. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1983, 76, 277–281. [Google Scholar] [CrossRef]
- Dua, R.; Sunkaria, A.; Kumar, V.; Gill, K.D. Impaired mitochondrial energy metabolism and kinetic properties of cytochrome oxidase following acute aluminium phosphide exposure in rat liver. Food Chem Toxicol. 2010, 48, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Price, N. Active exclusion of phosphine as a mechanism of resistance in Rhyzopertha dominica (F.)(Coleoptera: Bostrychidae). J. Stored Prod. Res. 1984, 20, 163–168. [Google Scholar] [CrossRef]
- Price, N. Comparison of the Uptake and Metabolism of 32 P-radiolabelled Phosphine in Susceptible and Resistant Strains of the Lesser Grain Borer (Rhyzopertha Dominica); MPKV: Maharastra, India, 1981; Volume 69. [Google Scholar]
- Dyte, C.; Mills, K.; Price, N. Recent work on fumigant-resistant insect strains. In Proceedings of the 6th British Pest Control Conference, Cambridge, UK, 7–10 September 1983; pp. 7–10. [Google Scholar]
- Bond, E.; Monro, H. The role of oxygen in the toxicity of fumigants to insects. J. Stored Prod. Res. 1967, 3, 295–310. [Google Scholar] [CrossRef]
- Gibbs, A.; Pomonis, J.G. Physical properties of insect cuticular hydrocarbons: The effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 112, 243–249. [Google Scholar] [CrossRef]
- Hadley, N.F. Cuticle: Ecological significance. In Biology of the Integument; Springer: Basel, Switzerland, 1984; pp. 685–693. [Google Scholar]
- El-Sayed, G.N.; Ignoffo, C.M.; Leathers, T.D. Effects of cuticle source and concentration on germination of conidia of two isolates of Nomuraea rileyi. Mycopathologia 1991, 113, 95–102. [Google Scholar] [CrossRef]
- Nelson, D.R.; Sukkestad, D.R. Normal and branched aliphatic hydrocarbons from the eggs of the tobacco hornworm. Biochemistry 1970, 9, 4601–4611. [Google Scholar] [CrossRef]
- Howard, R.W.; Lord, J.C. Cuticular lipids of the booklouse, Liposcelis bostrychophila: Hydrocarbons, aldehydes, fatty acids, and fatty acid amides. J. Chem. Ecol. 2003, 29, 615–627. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnajim, I.; Agarwal, M.; Liu, T.; Li, B.; Du, X.; Ren, Y. Preliminary Study on the Differences in Hydrocarbons Between Phosphine-Susceptible and -Resistant Strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) Using Direct Immersion Solid-Phase Microextraction Coupled with GC-MS. Molecules 2020, 25, 1565. https://doi.org/10.3390/molecules25071565
Alnajim I, Agarwal M, Liu T, Li B, Du X, Ren Y. Preliminary Study on the Differences in Hydrocarbons Between Phosphine-Susceptible and -Resistant Strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) Using Direct Immersion Solid-Phase Microextraction Coupled with GC-MS. Molecules. 2020; 25(7):1565. https://doi.org/10.3390/molecules25071565
Chicago/Turabian StyleAlnajim, Ihab, Manjree Agarwal, Tao Liu, Beibei Li, Xin Du, and Yonglin Ren. 2020. "Preliminary Study on the Differences in Hydrocarbons Between Phosphine-Susceptible and -Resistant Strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) Using Direct Immersion Solid-Phase Microextraction Coupled with GC-MS" Molecules 25, no. 7: 1565. https://doi.org/10.3390/molecules25071565
APA StyleAlnajim, I., Agarwal, M., Liu, T., Li, B., Du, X., & Ren, Y. (2020). Preliminary Study on the Differences in Hydrocarbons Between Phosphine-Susceptible and -Resistant Strains of Rhyzopertha dominica (Fabricius) and Tribolium castaneum (Herbst) Using Direct Immersion Solid-Phase Microextraction Coupled with GC-MS. Molecules, 25(7), 1565. https://doi.org/10.3390/molecules25071565