Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of DIC Treatments
2.2. Analytical Composition
2.3. Electrophoretic Analysis
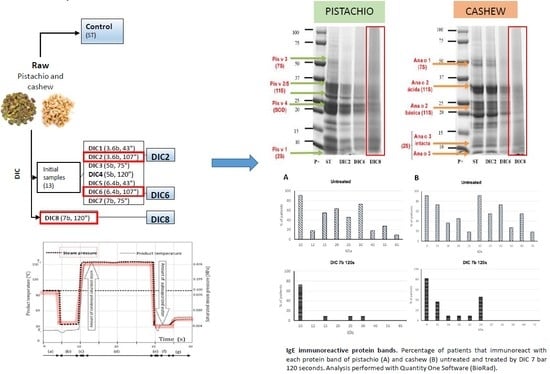
2.4. Immunodetection
2.4.1. Immunodetection with Anti-2S and Anti-11S IgG
2.4.2. Immunodetection with IgE from Human Sera
2.5. Proteins identification by LC/MS/MS
3. Materials and Methods
3.1. Plant Material
3.2. Human Sera
3.3. Controlled Instant Depressurization Treatments (DIC)
3.4. Protein Separation and Immunoblot
3.4.1. Immunodetection with IgG Antibodies
3.4.2. Immunodetection with IgE of Human Sera
3.5. Protein Identification by MS and Data Base Search
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojeda, P. Declaración Pública Sobre la Alergia a los Alimentos y la Anafilaxia. 2013. Available online: https://www.eaaci.org/attachments/FoodAllergy&AnaphylaxisPublicDeclarationSP.pdf (accessed on 3 March 2019).
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional composition of raw fresh cashew (Anacardium occidentale L.) kernels from different origin. Food Sci. Nutr. 2016, 4, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Himejima, M.; Kubo, I. Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J. Agric. Food Chem. 1991, 39, 418–421. [Google Scholar] [CrossRef]
- Dendena, B.; Corsi, S. Cashew, from seed to market: A review. Agron. Sustain. Dev. 2014, 34, 753–772. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.; Silva, I.; Vicente, A.A.; Oliveira, M.B.P.P.; Mafra, I. Pistachio nut allergy: An updated overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 546–562. [Google Scholar] [CrossRef] [Green Version]
- Ahn, K.; Bardina, L.; Grishina, G.; Beyer, K.; Sampson, H.A. Identification of two pistachio allergens, Pis v 1 and Pis v 2, belonging to the 2S albumin and 11S globulin family. Clin. Exp. Allergy 2009, 39, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Willison, L.N.; Tawde, P.; Robotham, J.M.; Penney, R.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Pistachio vicilin, Pis v 3, is immunoglobulin E-reactive and cross-reacts with the homologous cashew allergen, Ana o 1. Clin. Exp. Allergy 2008, 38, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Beyer, K.; Grishina, G.; Bardina, L.; Stalcup, D.; Sampson, H. Identification and cloning of 11S globulin, a new minor allergen from pistachio nut. Allergen Nomenclature Sub-Committee of the International Union of Immunological Societies, and to the EMBL/GenBank/DDBJ Databases. 2008. 36(Database issue): D25–D30. Available online: https:www.ncbi.nlm.nih.gov/sites/gquery (accessed on 17 April 2019).
- Ayuso, R.; Grishina, G.; Ahn, K. Identification of a MnSOD-like protein as a new major pistachio allergen. J. Allergy Clin. Immunol. 2007, 119, s115. [Google Scholar] [CrossRef]
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Mendes, C.; Costa, J.; Vicente, A.A.; Oliveira, M.B.; Mafra, I. Cashew Nut Allergy: Clinical Relevance and Allergen Characterisation. Clin. Rev. Allergy Immunol. 2019, 57, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Ana o 2, a major cashew (Anacardium occidentale L.) nut allergen of the legumin family. Int. Arch. Allergy Immunol. 2003, 132, 27–39. [Google Scholar] [CrossRef]
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Tawde, P.; Sathe, S.K.; Roux, K.H. Ana o 1, a cashew (Anacardium occidentale L.) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002, 110, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robotham, J.M.; Wang, F.; Seamon, V.; Teuber, S.S.; Sathe, S.K.; Sampson, H.A.; Beyer, K.; Seavy, M.; Roux, K.H. Ana o 3, an important cashew nut (Anacardium occidentale L.) allergen of the 2S albumin family. J. Allergy Clin. Immunol. 2005, 115, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Effects of daily food processing on allergenicity. Crit. Rev. Food Sci. Nutr. 2017, 59, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, C.; Cheng, H.; Sanchiz, A.; Ballesteros, I.; Easson, M.; Grimm, C.C.; Dieguez, M.C.; Linacero, R.; Burbano, C.; Maleki, S.J. Influence of enzymatic hydrolysis on the allergenic reactivity of processed cashew and pistachio. Food Chem. 2018, 241, 372–379. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.M.; Varela, A.; Guillamon, E.; Muzquiz, M.; Crespo, J.F.; Rodriguez, J.; Burbano, C. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol. Nutr. Food Res. 2009, 53, 1462–1468. [Google Scholar] [CrossRef]
- Sanchiz, A.; Cuadrado, C.; Dieguez, M.C.; Ballesteros, I.; Rodriguez, J.; Crespo, J.F.; Cuevas, N.L.; Rueda, J.; Linacero, R.; Cabanillas, B.; et al. Thermal processing effects on the IgE-reactivity of cashew and pistachio. Food Chem. 2018, 245, 595–602. [Google Scholar] [CrossRef]
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.; Muzquiz, M.; Haddad, J.; Allaf, K.; Rodriguez, J.; Crespo, J.; Burbano, C. Effect of Instant Controlled Pressure Drop on IgE Antibody Reactivity to Peanut, Lentil, Chickpea and Soybean Proteins. Int. Arch. Allergy Immunol. 2011, 156, 397–404. [Google Scholar] [CrossRef]
- Takacs, K.; Guillamon, E.; Pedrosa, M.M.; Cuadrado, C.; Burbano, C.; Muzquiz, M.; Haddad, J.; Allaf, K.; Maczo, A.; Polgar, M.; et al. Study of the effect of instant controlled pressure drop (DIC) treatment on IgE-reactive legume-protein patterns by electrophoresis and immunoblot. Food Agric. Immunol. 2014, 25, 173–185. [Google Scholar] [CrossRef]
- Haddad, J.; Allaf, K. A study of the impact of instantaneous controlled pressure drop on the trypsin inhibitors of soybean. J. Food Eng. 2007, 79, 353. [Google Scholar] [CrossRef]
- Haddad, J.; Louka, N.; Gadouleau, M.; Juhel, F.; Allaf, K. Application du nouveau procédé de séchage/texturation par Détente Instantanée Contrôlée DIC aux poissons: Impact sur les caractéristiques physico-chemiques du produit fini. Sci. Aliment. 2001, 21, 481–498. [Google Scholar] [CrossRef]
- Thomas, K.; Herouet-Guicheney, C.; Ladics, G.; Bannon, G.; Cockburn, A.; Crevel, R.; Fitzpatrick, J.; Mills, C.; Privalle, L.; Vieths, S. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food Chem. Toxicol. 2007, 45, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J. Food processing: Effects on allergenicity. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Sanchiz, A.; Pedrosa, M.M.; Guillamón, E.; Arribas, C.; Cabellos, B.; Linacero, R.; Cuadrado, C. Influence of boiling and autoclave processing on the phenolic content, antioxidant activity and functional properties of pistachio, cashew and chestnut flours. LWT 2019, 105, 250–256. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; William, H., Ed.; AOAC International: Rockville/Gaithersburg, MD, USA, 2003. [Google Scholar]
- Cabanillas, B.; Cuadrado, C.; Rodriguez, J.; Hart, J.; Burbano, C.; Crespo, J.F.; Novak, N. Potential changes in the allergenicity of three forms of peanut after thermal processing. Food Chem. 2015, 183, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Jenkins, J.; Marigheto, N.; Belton, P.; Gunning, A.; Morris, V. Allergens of the cupin superfamily. Biochem. Soc. Trans. 2002, 30, 925–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillamón, E.; Burbano, C.; Cuadrado, C.; Muzquiz, M.; Pedrosa, M.M.; Sanchez, M.; Cabanillas, B.; Crespo, J.F.; Rodriguez, J.; Haddad, J.; et al. Effect of an instantaneous controlled pressure drop on in vitro allergenicity to lupins (Lupinus albus var Multolupa). Int. Arch. Allergy Immunol. 2008, 145, 9–14. [Google Scholar] [CrossRef]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 821–830. [Google Scholar] [CrossRef]
- Blanco, D.; De Las Cuevas, N.; Barranco, R.; Fernandez Crespo, J.F.; Dieguez, M.C. Effects of Pressure and Thermal Processing on Cashew and Pistachio in vitro Allergic Reactivity. J. Allergy Clin. Immunol. 2018, 141 (Suppl. 2), AB243. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.; Mackie, A.; Burney, P.; Beyer, K.; Frewer, L.; Madsen, C.; Botjes, E.; Crevel, R.; van Ree, R. The prevalence, cost and basis of food allergy across Europe. Allergy 2007, 62, 717–722. [Google Scholar] [CrossRef]
- Lange, L.; Lasota, L.; Finger, A.; Vlajnic, D.; Büsing, S.; Meister, J.; Broekaert, I.; Pfannenstiel, C.; Friedrichs, F.; Price, M.; et al. Ana o 3-specific IgE is a good predictor for clinically relevant cashew allergy in children. Allergy 2017, 72, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Díaz, C.; Martín-Pedraza, L.; Benedé, S.; Haroun-Díaz, E.; de las Heras, M.; Batanero, E.; Cuesta-Herranz, J.; Villalba, M. Seed storage 2S albumins are predictive indicators of exclusive Anacardiaceae cross-reactivity. Clin. Exp. Allergy 2019, 49, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Haberstok, J.; Bieber, T.; Allam, J.-P. The immune privilege of the oral mucosa. Trends Mol. Med. 2008, 14, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Roberts, G.; Worm, M.; Bilo, M.B.; Brockow, K.; Fernandez Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of. Bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Sechi, S.; Chait, T. Modification of Cysteine residues by alkilation. A tool in peptide mapping and protein identification. Anal. Chem. 1998, 70, 5150–5158. [Google Scholar] [CrossRef]
Sample Availability: Samples of the untreated and DIC treated pistachio and cashew flours are available from the authors. |




| Sample | Total Protein (LECO) (g/100 g dm) | Total Protein (RC-DC) (g/100 g dm) | Soluble Protein (Bradford) (g/100 g dm) |
|---|---|---|---|
| Pistachio Raw | 39.50 ± 0.14 a * | 54.86 ± 0.00 a | 21.47 ± 1.24 a |
| Pistachio DIC2 | 39.13 ± 0.01 a | 57.5 ± 3.17 a | 10.69 ± 1.14 b |
| Pistachio DIC6 | 39.86 ± 0.28 ab | 37.99 ± 0.97 b | 5.84 ± 1.17 c |
| Pistachio DIC8 | 38.48 ± 0.26 b | 30.91 ± 0.40 c | 3.86 ± 0.67 c |
| Çashew Raw | 35.43 ± 0.13 a | 55.04 ± 3.35 a | 23.24 ± 0.24 a |
| Cashew DIC2 | 35.59 ± 0.38 a | 47.11 ± 1.06 b | 14.54 ± 0.33 b |
| Cashew DIC6 | 33.74 ± 0.17 b | 33.2 ± 0.40 c | 2.80 ± 0.11 c |
| Cashew DIC8 | 37.50 ± 0.18 c | 37.82 ± 1.50 c | 6.50 ± 0.14 d |
| Patient | Age/Sex | IgE Pistachio (kU/L) | IgE Cashew (kU/L) | Symptoms |
|---|---|---|---|---|
| P1 | 9/M | >100 | >100 | Anaphylaxis |
| P2 | 19/F | >100 | >100 | SWPC |
| P3 | 6/F | 71 | 70.1 | SWPC |
| P4 | 10/M | 76.2 | 75.4 | TS |
| P5 | 4/M | 74.4 | 74.5 | SWPC |
| P6 | 5/M | 33.2 | 39.9 | Urticaria, ES, C, D |
| P7 | 11/F | 16.4 | 10.1 | SWPC |
| P8 | 7/F | 15.4 | 10.6 | SWPC * |
| P9 | 5/F | 29.3 | 23.4 | Pruritus |
| P10 | 7/F | 19.9 | 13.1 | Anaphylaxis |
| P11 | 6/F | 18.0 | 11.3 | Urticaria, LS, ES |
| Sample | Pressure (bar) | Time (s) |
|---|---|---|
| ST | -- | -- |
| DIC1 | 3.6 | 43 |
| DIC2 | 3.6 | 107 |
| DIC3 | 5 | 75 |
| DIC4 | 5 | 120 |
| DIC5 | 6.4 | 43 |
| DIC6 | 6.4 | 107 |
| DIC7 | 7 | 75 |
| DIC8 | 7 | 120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, F.; Sanchiz, A.; Rodríguez-Pérez, R.; Pedrosa, M.; Quirce, S.; Haddad, J.; Besombes, C.; Linacero, R.; Allaf, K.; Cuadrado, C. Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts. Molecules 2020, 25, 1742. https://doi.org/10.3390/molecules25071742
Vicente F, Sanchiz A, Rodríguez-Pérez R, Pedrosa M, Quirce S, Haddad J, Besombes C, Linacero R, Allaf K, Cuadrado C. Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts. Molecules. 2020; 25(7):1742. https://doi.org/10.3390/molecules25071742
Chicago/Turabian StyleVicente, Fatima, Africa Sanchiz, Rosa Rodríguez-Pérez, Maria Pedrosa, Santiago Quirce, Joseph Haddad, Colette Besombes, Rosario Linacero, Karim Allaf, and Carmen Cuadrado. 2020. "Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts" Molecules 25, no. 7: 1742. https://doi.org/10.3390/molecules25071742
APA StyleVicente, F., Sanchiz, A., Rodríguez-Pérez, R., Pedrosa, M., Quirce, S., Haddad, J., Besombes, C., Linacero, R., Allaf, K., & Cuadrado, C. (2020). Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts. Molecules, 25(7), 1742. https://doi.org/10.3390/molecules25071742