Photodestruction of Diatomic Molecular Ions: Laboratory and Astrophysical Application
Abstract
:1. Introduction
2. Results and Discussion
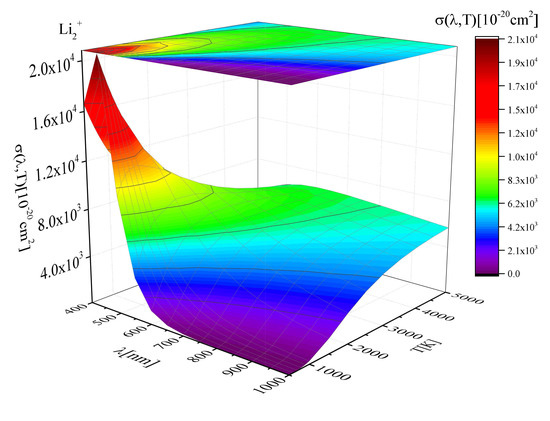
2.1. Cross-Sections
2.2. Rate Coefficients
2.3. Modeling
2.4. Further Usage
2.5. Development
3. The Method and Calculated Quantities
3.1. Photodissociation Cross-Sections
3.2. Photodissociation Spectral Rate Coefficient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jraij, A.; Allouche, A.R.; Magnier, S.; Aubert-Frécon, M. Theoretical spin-orbit structure of the alkali dimer cation K2+. Can. J. Phys. 2008, 86, 1409–1415. [Google Scholar] [CrossRef]
- Rabli, D.; Mccarroll, R. The low-lying adiabatic states of the K2+ alkali dimer. Chem. Phys. Lett. 2019, 723, 82–88. [Google Scholar] [CrossRef]
- Magnier, S.; Rousseau, S.; Allouche, A.R.; Hadinger, G.; Aubert-Frécon, M. Potential energy curves of 58 states of Li2+. Chem. Phys. 1999, 246, 57–64. [Google Scholar] [CrossRef]
- Jasik, P.; Wilczyński, J.; Sienkiewicz, J.E. Calculation of adiabatic potentials of Li2+. Eur. Phys. J. ST 2007, 144, 85–91. [Google Scholar] [CrossRef]
- Ignjatović, L.M.; Mihajlov, A.A. UV emission/absorption in H+A+ and H++A ion-atom collisions. 19th Summer Sch. Int. Symp. Phys. Ioniz. Gases Contrib. Pap. 1998, 1, 163–166. [Google Scholar]
- Ignjatović, L.M.; Mihajlov, A.A. Rate coefficient for the chemi-ionization in slow Li*(n)+Li and Na*(n)+Na collisions. Phys. Rev. A 2005, 72, 022715. [Google Scholar] [CrossRef]
- Ignjatović, L.M.; Mihajlov, A.A.; Klyucharev, A.N. The rate coefficients of the chemi-ionization processes in slow Li*(n) + Na collisions. J. Phys. B 2008, 41, 025203. [Google Scholar] [CrossRef]
- Mihajlov, A.A.; Srećković, V.A.; Ignjatović, L.M.; Klyucharev, A.N. The Chemi-Ionization Processes in Slow Collisions of Rydberg Atoms with Ground State Atoms: Mechanism and Applications. J. Clust. Sci. 2012, 23, 47–75. [Google Scholar] [CrossRef]
- Efimov, D.K.; Bruvelis, M.; Bezuglov, N.N.; Dimitrijević, M.S.; Klyucharev, A.N.; Srećković, V.A.; Gnedin, Y.N.; Fuso, F. Nonlinear Spectroscopy of Alkali Atoms in Cold Medium of Astrophysical Relevance. Atoms 2017, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Ignjatović, L.M.; Srećković, V.; Dimitrijević, M. Photoionization of the alkali molecular ions in geo-cosmical plasmas. Contrib. Astron. Obs. Skaln. Pleso 2020, 50, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Klyucharev, A.N.; Bezuglov, N.N.; Matveev, A.A.; Mihajlov, A.A.; Ignjatović, L.M.; Dimitrijević, M.S. Rate coefficients for the chemi-ionization processes in sodium- and other alkali-metal geocosmical plasmas. New Astr. Rev. 2007, 51, 547–562. [Google Scholar] [CrossRef]
- Rai, A.K.; Pati, J.K.; Parigger, C.G.; Dubey, S.; Rai, A.K.; Bhagabaty, B.; Mazumdar, A.C.; Duorah, K. The Plasma Spectroscopic Study of Dergaon Meteorite, India. Molecules 2020, 25, 984. [Google Scholar] [CrossRef] [Green Version]
- Beuc, R.; Peach, G.; Movre, M.; Horvatić, B. Lithium, sodium and potassium resonance lines pressure broadened by helium atoms. Astron. Astrophys. Trans. 2018, 30, 315–322. [Google Scholar]
- Sharp, C.M.; Burrows, A. Atomic and molecular opacities for brown dwarf and giant planet atmospheres. Astrophys. J. Suppl. Ser. 2007, 168, 140. [Google Scholar] [CrossRef] [Green Version]
- Bezuglov, N.; Klucharev, A.; Taratin, B.; Stacewicz, T.; Molisch, A.; Fuso, F.; Allegrini, M. Radiation trapping in an alkali-vapor-noble-gas mixture excited by a strong laser pulse. Opt. Commun. 1995, 120, 249–256. [Google Scholar] [CrossRef]
- Levine, J.S. The photochemistry of the early atmosphere. In The Photochemistry of Atmospheres; Academic Press: Orlando, FL, USA, 1985; pp. 3–38. [Google Scholar]
- Watanabe, A.; Dutta, C.; Nordlander, P.; Kimura, M.; Dalgarno, A. Charge-transfer cross sections for radiative charge transfer in Na + H+ and K + H+ collisions at very low energies. Phys. Rev. A 2002, 66, 044701. [Google Scholar] [CrossRef]
- Stwalley, W.C.; Zemke, W.T. Spectroscopy and structure of the Lithium Hydride diatomic molecules and ions. J. Phys. Chem. Ref. Data 1993, 22, 87–112. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Qu, Y.; Liu, C.; Wang, J.; Buenker, R.J. Ab initio many-electron study for the low-lying states of the alkali hydride cations in the adiabatic representation. J. Chem. Phys. 2012, 136, 124304. [Google Scholar]
- Beuc, R.; Movre, M.; Horvatić, B. Time-efficient numerical simulation of diatomic molecular spectra. Eur. Phys. J. D 2014, 68, 59. [Google Scholar] [CrossRef]
- Parigger, C.G.; Helstern, C.M.; Jordan, B.S.; Surmick, D.M.; Splinter, R. Laser-Plasma Spatiotemporal Cyanide Spectroscopy and Applications. Molecules 2020, 25, 615. [Google Scholar] [CrossRef] [Green Version]
- Parigger, C.G.; Helstern, C.M.; Jordan, B.S.; Surmick, D.M.; Splinter, R. Laser-Plasma Spectroscopy of Hydroxyl with Applications. Molecules 2020, 25, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beuc, R.; Pichler, G. High-Temperature Optical Spectra of Diatomic Molecules: Influence of the Avoided Level Crossing. Atoms 2020, 8, 28. [Google Scholar] [CrossRef]
- Pichler, G.; Beuc, R.; Kokaj, J.; Sarkisyan, D.; Jose, N.; Mathew, J. Photoionization of KCs Molecule: Origin of the Structured Continuum? Atoms 2020, 8, 24. [Google Scholar] [CrossRef]
- Pichler, G.; Makdisi, Y.; Kokaj, J.; Mathew, J.; Rakić, M.; Beuc, R. Superheating effects in line broadening of dense alkali vapors. J. Phys. Conf. Ser. 2017, 810, 012013. [Google Scholar] [CrossRef] [Green Version]
- Ignjatović, L.M.; Mihajlov, A.A.; Srećković, V.A.; Dimitrijević, M.S. Absorption non-symmetric ion-atom processes in helium-rich white dwarf atmospheres. Mon. Not. R. Astron. Soc. 2014, 439, 2342–2350. [Google Scholar] [CrossRef] [Green Version]
- Albert, D.; Antony, B.K.; Ba, Y.A.; Babikov, Y.L.; Bollard, P.; Boudon, V.; Delahaye, F.; Del Zanna, G.; Dimitrijević, M.S.; Drouin, B.J.; et al. A decade with VAMDC: Results and ambitions. Atoms 2020, 8, 76. [Google Scholar] [CrossRef]
- Fontenla, J.M.; Avrett, E.; Thuillier, G.; Harder, J. Semiempirical Models of the Solar Atmosphere. I. The Quiet- and Active Sun Photosphere at Moderate Resolution. Astrophys. J. 2006, 639, 441–458. [Google Scholar] [CrossRef] [Green Version]
- Hauschildt, P.; Baron, E. Cool stellar atmospheres with PHOENIX. Mem. Della Soc. Astron. Ital. Suppl. 2005, 7, 140. [Google Scholar]
- Strobel, D.F.; Zhu, X.; Summers, M.E. On the vertical thermal structure of Io’s atmosphere. Icarus 1994, 111, 18–30. [Google Scholar] [CrossRef]
- Moses, J.I.; Zolotov, M.Y.; Fegley, B., Jr. Photochemistry of a volcanically driven atmosphere on Io: Sulfur and oxygen species from a Pele-type eruption. Icarus 2002, 156, 76–106. [Google Scholar] [CrossRef]
- Wilson, J.K.; Mendillo, M.; Baumgardner, J.; Schneider, N.M.; Trauger, J.T.; Flynn, B. The dual sources of Io’s sodium clouds. Icarus 2002, 157, 476–489. [Google Scholar] [CrossRef] [Green Version]
- Mendillo, M.; Baumgardner, J.; Flynn, B.; Hughes, W.J. The extended sodium nebula of Jupiter. Nature 1990, 348, 312–314. [Google Scholar] [CrossRef]
- Schaefer, L.; Fegley, B., Jr. Alkali and halogen chemistry in volcanic gases on Io. Icarus 2005, 173, 454–468. [Google Scholar] [CrossRef]
- Li, H.; Aoki, W.; Matsuno, T.; Kumar, Y.B.; Shi, J.; Suda, T.; Zhao, G. Enormous Li Enhancement Preceding Red Giant Phases in Low-mass Stars in the Milky Way Halo. Astrophys. J. Lett. 2018, 852, L31. [Google Scholar]
- Marinković, B.P.; Vujčić, V.; Sushko, G.; Vudragović, D.; Marinković, D.B.; Đorđević, S.; Ivanović, S.; Nešić, M.; Jevremović, D.; Solov’yov, A.V.; et al. Development of collisional data base for elementary processes of electron scattering by atoms and molecules. Nucl. Instrum. Methods Phys. Res. 2015, 354, 90–95. [Google Scholar] [CrossRef]
- Vujčič, V.; Jevremović, D.; Mihajlov, A.A.; Ignjatović, L.M.; Srećković, V.A.; Dimitrijević, M.S.; Malović, M. MOL-D: A Collisional Database and Web Service within the Virtual Atomic and Molecular Data Center. J. Astrophys. Astron. 2015, 36, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Jevremović, D.; Dimitrijević, M.; Popović, L.; Dačić, M.; Benišek, V.P.; Bon, E.; Gavrilović, N.; Kovačević, J.; Benišek, V.; Kovačević, A.; et al. The project of Serbian Virtual Observatory and data for stellar atmosphere modeling. New Astron. Rev. 2009, 53, 222–226. [Google Scholar] [CrossRef]
- Srećković, V.A.; Jevremović, D.; Vujčić, V.; Ignjatović, L.M.; Milovanović, N.; Erkapić, S.; Dimitrijević, M.S. Mol-D a database and a web service within the Serbian virtual observatory and the virtual atomic and molecular data centre. Proc. Int. Astron. Union 2016, 12, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Milosavljević, A.R.; Nicolas, C.; Lemaire, J.; Dehon, C.; Thissen, R.; Bizau, J.M.; Réfrégiers, M.; Nahon, L.; Giuliani, A. Photoionization of a protein isolated in vacuo. Phys. Chem. Chem. Phys. 2011, 13, 15432–15436. [Google Scholar] [CrossRef]
- Ranković, M.L.; Cerovski, V.Z.; Canon, F.; Nahon, L.; Giuliani, A.; Milosavljević, A.R. Photodissociation of protonated Leucine-Enkephalin peptide in the VUV range. In Proceedings of the 29th International Conference on Photonic, Electronic, and Atomic Collisions (ICPEAC), Deauville, France, 23–30 July 2019; IOP Publishing: Bristol, UK, 2019; Volume 635. [Google Scholar]
- Giuliani, A.; Milosavljević, A.R.; Canon, F.; Nahon, L. Contribution of synchrotron radiation to photoactivation studies of biomolecular ions in the gas phase. Mass Spectrom. Rev. 2014, 33, 424–441. [Google Scholar] [CrossRef]
- Baiano, C.; Lupi, J.; Tasinato, N.; Puzzarini, C.; Barone, V. The role of state-of-the-art quantum-chemical calculations in astrochemistry: Formation route and spectroscopy of ethanimine as a paradigmatic case. Molecules 2020, 25, 2873. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Vishakantaiah, J.; Meka, J.K.; Sivaprahasam, V.; Chandrasekaran, V.; Thombre, R.; Thiruvenkatam, V.; Mallya, A.; Rajasekhar, B.N.; Muruganantham, M.; et al. Shock Processing of Amino Acids Leading to Complex Structures-Implications to the Origin of Life. Molecules 2020, 25, 5634. [Google Scholar] [CrossRef] [PubMed]
- Srećković, V.A.; Mihajlov, A.A.; Ignjatović, L.M.; Dimitrijević, M.S. Ion-atom radiative processes in the solar atmosphere: Quiet Sun and sunspots. Adv. Space Res. 2014, 54, 1264–1271. [Google Scholar] [CrossRef] [Green Version]
- Mihajlov, A.A.; Jevremović, D.; Hauschildt, P.; Dimitrijević, M.S.; Ignjatović, L.M.; Alard, F. Influence of chemi-ionization and chemi-recombination processes on hydrogen line shapes in M dwarfs. Astron. Astrophys. 2007, 471, 671–673. [Google Scholar] [CrossRef] [Green Version]
- Srećković, V.A.; Ignjatović, L.M.; Jevremović, D.; Vujčić, V.; Dimitrijević, M.S. Radiative and Collisional Molecular Data and Virtual Laboratory Astrophysics. Atoms 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Mihajlov, A.; Sakan, N.; Srećković, V.; Vitel, Y. Modeling of continuous absorption of electromagnetic radiation in dense partially ionized plasmas. J. Phys. A 2011, 44, 095502. [Google Scholar] [CrossRef] [Green Version]





| [nm] | |||||||||
| 400 | 115.794 | −83.441 | 11.3946 | 41.7324 | −43.2074 | 5.88338 | 75.7089 | −61.4123 | 8.36583 |
| 500 | 110.628 | −80.5359 | 10.9971 | 82.4755 | −65.1067 | 8.8713 | 85.8781 | −66.8371 | 9.10941 |
| 600 | 97.0323 | −73.0543 | 9.96852 | 80.6419 | −64.0402 | 8.72452 | 76.0451 | −61.3987 | 8.36012 |
| 700 | 84.114 | −65.944 | 8.98853 | 72.9253 | −59.7686 | 8.13487 | 64.6813 | −55.126 | 7.49352 |
| 800 | 72.9344 | −59.7847 | 8.13795 | 64.6227 | −55.1811 | 7.50068 | 54.2741 | −49.373 | 6.69625 |
| 900 | 63.4083 | −54.5306 | 7.41114 | 56.8894 | −50.9076 | 6.90904 | 45.2003 | −44.3536 | 5.99965 |
| 1000 | 55.2672 | −50.0354 | 6.7884 | 49.9549 | −47.0722 | 6.37719 | 37.3453 | −40.0034 | 5.39484 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srećković, V.A.; Ignjatović, L.M.; Dimitrijević, M.S. Photodestruction of Diatomic Molecular Ions: Laboratory and Astrophysical Application. Molecules 2021, 26, 151. https://doi.org/10.3390/molecules26010151
Srećković VA, Ignjatović LM, Dimitrijević MS. Photodestruction of Diatomic Molecular Ions: Laboratory and Astrophysical Application. Molecules. 2021; 26(1):151. https://doi.org/10.3390/molecules26010151
Chicago/Turabian StyleSrećković, Vladimir A., Ljubinko M. Ignjatović, and Milan S. Dimitrijević. 2021. "Photodestruction of Diatomic Molecular Ions: Laboratory and Astrophysical Application" Molecules 26, no. 1: 151. https://doi.org/10.3390/molecules26010151
APA StyleSrećković, V. A., Ignjatović, L. M., & Dimitrijević, M. S. (2021). Photodestruction of Diatomic Molecular Ions: Laboratory and Astrophysical Application. Molecules, 26(1), 151. https://doi.org/10.3390/molecules26010151