Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes
Abstract
:1. Introduction
2. Results and Discussion
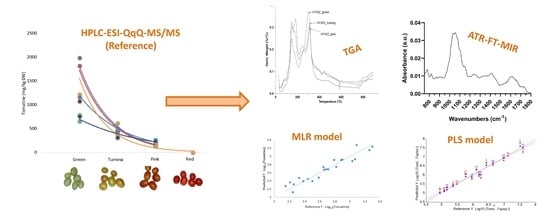
2.1. HPLC-ESI-QqQ-MS/MS Glycoalkaloid Determination in Different Industrial Tomato Varieties at Different Vine-Ripe Stages
2.2. Thermogravimetric Analysis (TGA)
2.3. Attenuated Total Reflection–Fourier Transform Mid-Infrared Spectroscopy (ATR-FT-MIR)
2.4. Chemometric Approach
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. HPLC-ESI-QqQ-MS/MS Quantification of Glycoalkaloids
3.4. Thermogravimetric Analysis (TGA)
3.5. Attenuated Total Reflection–Fourier Transform Mid-Infrared Spectroscopy (ATR-FT-MIR)
3.6. Multiple Linear Regression (MLR)
3.7. Chemometric Approach
3.7.1. Data Processing
3.7.2. Spectral Pre-Processing
3.7.3. Partial Least Squares Regression (PLSR) and Martens’ Uncertainty Test
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kim, H.J.; Lee, I.S.; Byun, J.O.; Kozukue, N. Tomatine-containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J. Agric. Food Chem. 2009, 57, 5727–5733. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef]
- Liu, J.; Kanetake, S.; Wu, Y.H.; Tam, C.; Cheng, L.W.; Land, K.M.; Friedman, M. Antiprotozoal effects of the tomato tetrasaccharide glycoalkaloid tomatine and the aglycone tomatidine on mucosal trichomonads. J. Agric. Food Chem. 2016, 64, 8806–8810. [Google Scholar] [CrossRef]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Pardini, A.; Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Effect of different post-harvest storage conditions and heat treatment on tomatine content in commercial varieties of green tomatoes. J. Food Comp. Anal. 2021, 96, 103735. [Google Scholar] [CrossRef]
- Serratì, S.; Porcelli, L.; Guida, S.; Ferretta, A.; Iacobazzi, R.M.; Cocco, T.; Maida, I.; Tamasi, G.; Rossi, C.; Manganelli, M.; et al. Tomatine displays antitumor potential in in vitro models of metastatic melanoma. Int. J. Mol. Sci. 2020, 21, 5243. [Google Scholar] [CrossRef]
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical characterisation and antihypertensive effects of locular gel and serum of Lycopersicum esculentum L. var. “Camone” tomato in Spontaneously Hypertensive Rats. Molecules 2020, 25, 3758. [Google Scholar] [CrossRef]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 2019, 7, 2907–2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites-potential benefits for plant and human health. Crit. Rev. Plant. Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonechi, C.; Lamponi, S.; Donati, A.; Tamasi, G.; Consumi, M.; Leone, G.; Rossi, C.; Magnani, A. Effect of resveratrol on platelet aggregation by fibrinogen protection. Biophys. Chem. 2017, 222, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Leicach, S.R.; Chludil, H.D. Plant secondary metabolites: Structure–activity relationships in human health prevention and treatment of common diseases. Stud. Nat. Prod. Chem. 2014, 42, 267–304. [Google Scholar]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar]
- Liao, B.; Chen, X.; Han, J.; Dan, Y.; Wang, L.; Jiao, W.; Song, J.; Chen, S. Identification of commercial Ganoderma (Lingzhi) species by ITS2 sequences. Chin. Med. 2015, 10, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Pei, Y.; Wu, L.; Zhang, Q.; Wang, Y. Geographical traceability of cultivatedParis polyphylla var. yunnanensis using ATR-FTMIR spectroscopy with three mathematical algorithms. Anal. Methods 2019, 11, 113–122. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.V.; Gomez, X.; Gonzalez-Andres, F.; Moran, A. Characterization of different compost extracts using fourier-transform infrared spectroscopy (FTIR) and thermal analysis. Biodegradation 2008, 19, 815–830. [Google Scholar] [CrossRef]
- Durazzo, A.; Kiefer, J.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Aguzzi, A.; Gambelli, L.; Lisciani, S.; Marletta, L. Qualitative analysis of traditional italian dishes: FTIR approach. Sustainability 2018, 10, 4112. [Google Scholar] [CrossRef] [Green Version]
- Bunghez, I.R.; Raduly, M.; Doncea, S.; Aksahin, I.; Ion, R.M. Lycopene determination in tomatoes by different spectral techniques (UV-VIS, FTIR and HPLC). Digest J. Nanomater. Biostruct. 2011, 6, 1349–1356. [Google Scholar]
- Tan, C.P.; Che Man, Y.B. Comparative differential scanning calorimetric analysis of vegetable oils: I. Effects of heating rate variation. Phytochem. Anal. 2002, 13, 129–141. [Google Scholar] [CrossRef]
- Kotti, F.; Chiavaro, E.; Cerretani, L.; Barnaba, C.; Gargouri, M.; Bendini, A. Chemical and thermal characterization of Tunisian extra virgin olive oil from Chetoui and Chemlali cultivarsand different geographical origin. Eur. Food Res. Technol. 2009, 228, 735–742. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Xu, X.; Jin, Z. Starch retrogradation studied by thermogravimetric analysis (TGA). Carbohydr. Polym. 2011, 84, 1165–1168. [Google Scholar] [CrossRef]
- Manara, P.; Vamvuka, D.; Sfakiotakis, S.; Vanderghem, C.; Richel, A.; Zabaniotou, A. Mediterranean agri-food processing wastes pyrolysis after pre-treatment and recovery of precursor materials: A TGA-based kinetic modeling study. Food Res. Int. 2015, 73, 44–51. [Google Scholar] [CrossRef]
- Kozukue, N.; Friedman, M. Tomatine, chlorophyll, β-carotene and lycopene content in tomatoes during growth and maturation. J. Sci. Food Agric. 2003, 83, 195–200. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Franzi, C.; Tamasi, G.; Lamponi, S.; Donati, A.; Magnani, A.; Rossi, C.; Bonechi, C. Development of liposomal formulations to potentiate natural lovastatin inhibitory activity towards 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase. J. Drug Deliv. Sci. Technol. 2018, 43, 107–112. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Pepi, S.; Lamponi, S.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Alginate-gelatin formulation to modify lovastatin release profile from red yeast rice for hypercholesterolemia therapy. Ther. Deliv. 2017, 8, 843–854. [Google Scholar] [CrossRef]
- Khiari, B.; Moussaoui, M.; Jeguirim, M. Tomato-processing by-product combustion: Thermal and kinetic analyses. Materials 2019, 12, 553. [Google Scholar] [CrossRef] [Green Version]
- Vega, D.; Villar, M.A.; Failla, M.D.; Vallés, E.M. Thermogravimetric analysis of starch-based biodegradable blends. Polym. Bull. 1996, 37, 229–235. [Google Scholar] [CrossRef]
- De Nardo, T.; Shiroma-Kian, C.; Halim, Y.; Francis, D.; Rodriguez-Saona, L.E. Rapid and simultaneous determination of lycopene and β-carotene contents in tomato juice by infrared spectroscopy. J. Agric. Food Chem. 2009, 57, 1105–1112. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant. Sci. 2014, 5, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Yap, K.Y.L.; Chan, S.Y.; Lim, C.S. Infrared-based protocol for the identification and categorization of ginseng and its products. Food Res. Int. 2007, 40, 643–652. [Google Scholar] [CrossRef]
- Osborne, J.W.; Costello, A.B. Sample size and subject to item ratio in principal components analysis. Pract. Asses. Res. Eval. 2004, 9, 11–20. [Google Scholar]
- USDA. United States Standards for Grades of Fresh Tomatoes; United States Department of Agriculture: Washington, DC, USA, 1997.
- Leone, G.; Consumi, M.; Lamponi, S.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Thixotropic PVA hydrogel enclosing a hydrophilic PVP core as nucleus pulposus substitute. Mater. Sci. Eng. C 2019, 98, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Donati, A.; Lamponi, S.; Rossi, C.; Magnani, A. Enriched gellan gum hydrogel as visco-supplement. Carbohydr. Polym. 2020, 227, 115347. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jares, C.M.; Médina, B. Application of multivariate calibration to the simultaneous routine determination of ethanol, glycerol, fructose, glucose and total residual sugars in botrytized-grape sweet wines by means of near-infrared reflectance spectroscopy. Fresenius J. Anal. Chem. 1997, 357, 86–91. [Google Scholar] [CrossRef]
- Oliveri, P. Class-modelling in food analytical chemistry: Development, sampling, optimisation and validation issues. A tutorial. Anal. Chim.Acta 2017, 982, 9–19. [Google Scholar] [CrossRef]
- Shah, N.; Cynkar, W.; Smith, P.; Cozzolino, D. Use of attenuated total reflectance midinfrared for rapid and real-time analysis of compositional parameters in commercial white grape juice. J. Agric. Food Chem. 2010, 58, 3279–3283. [Google Scholar] [CrossRef]
- Li, B.; Martin, E.; Morris, J. Latent variable selection in partial least squares modelling. IFAC Proc. Vol. 2001, 34, 463–468. [Google Scholar] [CrossRef]
- Croce, R.; Malegori, C.; Oliveri, P.; Medici, I.; Cavaglioni, A.; Rossi, C. Prediction of quality parameters in straw wine by means of FT-IR spectroscopy combined with multivariate data processing. Food Chem. 2020, 305, 125512. [Google Scholar] [CrossRef]
- Silalahi, D.D.; Midi, H.; Arasan, J.; Mustafa, M.S.; Caliman, J.P. Robust generalized multiplicative scatter correction algorithm on pretreatment of near infrared spectral data. Vib. Spectrosc. 2018, 97, 55–65. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Forina, M.; Latenri, S.; Cerrato Oliveros, M.C.; Pizarro Millan, C. Selection of useful predictors in multivariate calibration. Anal. Bioanal. Chem. 2004, 380, 397–418. [Google Scholar] [CrossRef]







| Variety | Ripening Stage | α-Tomatine | Dehydrotomatine | Tomatine |
|---|---|---|---|---|
| H1015 | Green | 1028 ± 53 a | 147 ± 9 a | 1176 ± 54 a |
| Turning | 441 ± 28 b | 78 ± 8 b,c | 519 ± 29 b | |
| Pink | 180 ± 19 c,d,e | 34 ± 5 d,e | 213 ± 20 c,d | |
| H1301 | Green | 1614 ± 40 f | 215 ± 4 f | 1829 ± 41 e |
| Turning | 400 ± 17 b | 57 ± 3 g | 457 ± 17 b,f | |
| Pink | 141 ± 12 c | 21 ± 2 h | 161 ± 12 c,g | |
| H3402 | Green | 688 ± 40 g | 109 ± 11 i | 796 ± 41 h |
| Turning | 535 ± 19 h | 86 ± 7 c,j | 621 ± 20 i | |
| Pink | 120 ± 11 c | 21 ± 3 h | 141 ± 11 g | |
| H3406 | Green | 1772 ± 33 i | 221 ± 3 f | 1993 ± 33 j |
| Turning | 388 ± 18 b | 61 ± 4 g | 449 ± 18 f,k | |
| Pink | 155 ± 11 c,d | 26 ± 3 d,h | 181 ± 12 c,d,g | |
| H5108 | Green | 552 ± 45 h | 111 ± 7 i | 663 ± 46 i |
| Turning | 420 ± 27 b | 69 ± 7 b | 489 ± 28 b,f | |
| Pink | 238 ± 17 e,j | 42 ± 6 e | 279 ± 18 l,m | |
| H7204 | Green | 1088 ± 34 a | 143 ± 12 a | 1231 ± 36 a |
| Turning | 314 ± 16 k | 75 ± 6 b,c | 388 ± 17 k | |
| Pink | 148 ± 17 c | 28 ± 4 d,h | 176 ± 17 c,g | |
| Red | 14 ± 2 l | <LOD | 14 ± 2 n | |
| Lyco1 | Green | 678 ± 62 g | 90 ± 8 j | 768 ± 63 h |
| Turning | 294 ± 22 j,k | 26 ± 3 d,h | 320 ±22 m | |
| Pink | 217 ± 32 d,e | 25 ± 4 d,h | 242 ± 32 d,l | |
| Fokker | Green | 952 ± 101 m | 128 ± 15 k | 1080 ± 102 o |
| Variety | Ripening Stage | Weight Loss | |
|---|---|---|---|
| 120–200 °C | 200–400 °C | ||
| H1015 | Green | 17.5 ± 0.2 | 38.2 ± 0.3 |
| Turning | 19.0 ± 0.2 | 36.4 ± 0.2 | |
| Pink | 19.3 ± 0.2 | 34.4 ± 0.5 | |
| H1301 | Green | 15.9 ± 0.1 | 39.7 ± 0.8 |
| Turning | 19.0 ± 0.4 | 36.3 ± 0.3 | |
| Pink | 20.4 ± 0.3 | 33.8 ± 0.2 | |
| H3402 | Green | 16.9 ± 0.3 | 37.8 ± 0.4 |
| Turning | 17.5 ± 0.5 | 38 ± 0.1 | |
| Pink | 20.5 ± 0.1 | 33 ± 0.3 | |
| H3406 | Green | 14.9 ± 0.1 | 40.1 ± 0.2 |
| Turning | 18.5 ± 0.6 | 36.7 ± 0.3 | |
| Pink | 20.3 ± 0.2 | 33.3 ± 0.5 | |
| H5108 | Green | 18.4 ± 0.3 | 36.7 ± 0.6 |
| Turning | 18.7 ± 0.2 | 36.3 ± 0.5 | |
| Pink | 20.3 ± 0.4 | 34.6 ± 0.2 | |
| H7204 | Green | 14.1 ± 0.2 | 40.1 ± 0.3 |
| Turning | 18.9 ± 0.3 | 34.3 ± 0.4 | |
| Pink | 24.2 ± 0.3 | 33.2 ± 0.6 | |
| Lyco1 | Green | 16.9 ± 0.5 | 37.7 ± 0.4 |
| Turning | 19.9 ± 0.4 | 33.9 ± 0.6 | |
| Pink | 19.5 ± 0.2 | 33.7 ± 0.4 | |
| Fokker | Green | 16.8 ± 0.2 | 37.4 ± 0.5 |
| Wavenumber (cm−1) | Proposed Assignment |
|---|---|
| 1720 | C=O ester |
| 1650 | Amide I, β-sheet |
| 1604 | C–C aromatic |
| 1551 | C–C aromatic |
| 1520 | Amide II, C≡N stretching |
| 1410 | CH2 bending of lipids and fatty acids |
| 1350 | CH3 bending proteins and lipids and CH2 wagging and twisting |
| 1240 | OH bending |
| 1196 | C–O–C ester stretching |
| 1145 | C–O–C ester stretching |
| 1055 | C–O–C glycosidic bond |
| 955 | CH(trans OOP) |
| Variety | Ripening Stage | Tomatine | ||
|---|---|---|---|---|
| HPLC-ESI-QqQ-MS/MS Experimental | TGA/MLR Model Predicted | ATR-FT-MIR/PLS Model Predicted | ||
| H1015 | Green | 1176 ± 54 | 851 | 1177 |
| Turning | 519 ± 29 | 481 | 482 | |
| Pink | 213 ± 20 | 296 | 213 | |
| H1301 | Green | 1829 ± 41 | 1422 | 1820 |
| Turning | 457 ± 17 | 470 | 458 | |
| Pink | 161 ± 12 | 229 | 160 | |
| H3402 | Green | 796 ± 41 | 830 | 797 |
| Turning | 621 ± 20 | 813 | 621 | |
| Pink | 141 ± 11 | 189 | 144 | |
| H3406 | Green | 1993 ± 33 | 1735 | 1874 |
| Turning | 449 ± 18 | 544 | 454 | |
| Pink | 181 ± 12 | 207 | 182 | |
| H5108 | Green | 663 ± 46 | 550 | 664 |
| Turning | 489 ± 28 | 486 | 461 | |
| Pink | 279 ± 18 | 278 | 280 | |
| H7204 | Green | 1231 ± 36 | 1893 | 1182 |
| Turning | 388 ± 17 | 302 | 371 | |
| Pink | 176 ± 17 | 133 | 177 | |
| Lyco1 | Green | 768 ± 63 | 811 | 742 |
| Turning | 320 ±22 | 248 | 325 | |
| Pink | 242 ± 32 | 247 | 239 | |
| Fokker | Green | 1080 ± 102 | 766 | 1039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamasi, G.; Pardini, A.; Croce, R.; Consumi, M.; Leone, G.; Bonechi, C.; Rossi, C.; Magnani, A. Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes. Molecules 2021, 26, 3068. https://doi.org/10.3390/molecules26113068
Tamasi G, Pardini A, Croce R, Consumi M, Leone G, Bonechi C, Rossi C, Magnani A. Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes. Molecules. 2021; 26(11):3068. https://doi.org/10.3390/molecules26113068
Chicago/Turabian StyleTamasi, Gabriella, Alessio Pardini, Riccardo Croce, Marco Consumi, Gemma Leone, Claudia Bonechi, Claudio Rossi, and Agnese Magnani. 2021. "Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes" Molecules 26, no. 11: 3068. https://doi.org/10.3390/molecules26113068
APA StyleTamasi, G., Pardini, A., Croce, R., Consumi, M., Leone, G., Bonechi, C., Rossi, C., & Magnani, A. (2021). Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes. Molecules, 26(11), 3068. https://doi.org/10.3390/molecules26113068